- British Pharmacopoeia Volume I & II
- Monographs: Medicinal and Pharmaceutical Substances
Furazolidone |
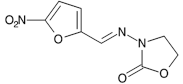
C8H7N3O5 225.2 67-45-8
Antiprotozoal; antibacterial.
Furazolidone is 3-(5-nitrofurfurylideneamino)oxazolidin-2-one. It contains not less than 97.0% and not more than 103.0% of C8H7N3O5, calculated with reference to the dried substance.
A yellow, crystalline powder.
Very slightly soluble in water and in ethanol (96%); practically insoluble in ether.
A. The infrared absorption spectrum, Appendix II A, is concordant with the reference spectrum of furazolidone (RS 164).
B. Dissolve 1 mg in 1 ml of dimethylformamide and add 0.05 ml of 1m ethanolic potassium hydroxide. A deep blue colour is produced.
Shake 1 g for 15 minutes with 100 ml of carbon dioxide-free water and filter. The pH of the filtrate is 4.5 to 7.0, Appendix V L.
Carry out in subdued light the method for thin-layer chromatography, Appendix III A, using silica gel G as the coating substance and a mixture of 95 volumes of toluene and 5 volumes of 1,4-dioxan as the mobile phase. Apply separately to the plate 20 µl of solution (1) and 10 µl of solution (2). For solution (1) dissolve 50 mg of the substance being examined in 5 ml of dimethylformamide by heating on a water bath for a few minutes, allow to cool and dilute to 10 ml with acetone. Solution (2) contains 0.010% w/v solution of nitrofurfural diacetate BPCRS in a mixture of equal volumes of dimethylformamide and acetone. After removal of the plate, heat it at 105° for 5 minutes and spray with a solution prepared by dissolving 0.75 g of phenylhydrazine hydrochloride in 10 ml of ethanol (96%), diluting to 50 ml with water, adding activated charcoal, filtering and then adding 25 ml of hydrochloric acid and sufficient water to produce 200 ml. Any spot corresponding to nitrofurfural diacetate in the chromatogram obtained with solution (1) is not more intense than the spot in the chromatogram obtained with solution (2).
When dried to constant weight at 105°, loses not more than 0.5% of its weight. Use 1 g.
Not more than 0.1%, Appendix IX A.
Carry out the following procedure protected from light. To 80 mg add 150 ml of dimethylformamide, swirl to dissolve and add sufficient water to produce 500 ml. Dilute 5 ml to 100 ml with water and mix. Measure the absorbance of the resulting solution at the maximum at 367 nm, Appendix II B. Calculate the content of C8H7N3O5 taking 750 as the value of A(1%, 1 cm) at the maximum at 367 nm.
Furazolidone should be protected from light.