- British Pharmacopoeia Volume I & II
- Monographs: Medicinal and Pharmaceutical Substances
Isoconazole Nitrate |
 |
(Ph. Eur. monograph 1017)
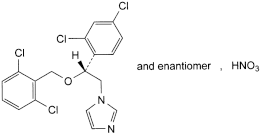
C18H14Cl4N2O,HNO3 479.1 24168-96-5
Antifungal.
Ph Eur
1-[(2RS)-2-[(2,6-Dichlorobenzyl)oxy]-2-(2,4-dichlorophenyl)ethyl]-1H-imidazole nitrate.
99.0 per cent to 101.0 per cent (dried substance).
White or almost white powder.
Very slightly soluble in water, soluble in methanol, slightly soluble in ethanol (96 per cent).
First identification A, B.
Second identification A, C, D.
A. Melting point (2.2.14): 178 °C to 182 °C.
B. Infrared absorption spectrophotometry (2.2.24).
Preparation Discs.
Comparison isoconazole nitrate CRS.
C. Thin-layer chromatography (2.2.27).
Test solution Dissolve 30 mg of the substance to be examined in methanol R and dilute to 5 mL with the same solvent.
Reference solution (a) Dissolve 30 mg of isoconazole nitrate CRS in methanol R and dilute to 5 mL with the same solvent.
Reference solution (b) Dissolve 30 mg of isoconazole nitrate CRS and 30 mg of econazole nitrate CRS in methanol R, then dilute to 5 mL with the same solvent.
Plate TLC octadecylsilyl silica gel plate R.
Mobile phase ammonium acetate solution R, dioxan R, methanol R (20:40:40 V/V/V).
Application 5 µL.
Development Over a path of 15 cm.
Drying In a current of warm air for 15 min.
Detection Expose to iodine vapour until the spots appear and examine in daylight.
System suitability Reference solution (b):
- — the chromatogram shows 2 clearly separated spots.
Results The principal spot in the chromatogram obtained with the test solution is similar in position, colour and size to the principal spot in the chromatogram obtained with reference solution (a).
D. It gives the reaction of nitrates (2.3.1).
Dissolve 0.20 g in methanol R and dilute to 20.0 mL with the same solvent.
Solution S is clear (2.2.1) and not more intensely coloured than reference solution Y7 (2.2.2, Method II).
- 0.10° to + 0.10°, determined on solution S.
Liquid chromatography (2.2.29).
Test solution Dissolve 0.100 g of the substance to be examined in the mobile phase and dilute to 10.0 mL with the mobile phase.
Reference solution (a) Dissolve 2.5 mg of isoconazole nitrate CRS and 2.5 mg of econazole nitrate CRS in the mobile phase, then dilute to 100.0 mL with the mobile phase.
Reference solution (b) Dilute 1.0 mL of the test solution to 100.0 mL with the mobile phase. Dilute 5.0 mL of this solution to 20.0 mL with the mobile phase.
- — size: l = 0.1 m, Ø = 4.6 mm;
- — stationary phase: octadecylsilyl silica gel for chromatography R (3 µm).
Mobile phase Dissolve 6.0 g of ammonium acetate R in a mixture of 300 mL of acetonitrile R, 320 mL of methanol R and 380 mL of water R.
Flow rate 2 mL/min.
Detection Spectrophotometer at 235 nm.
Equilibration With the mobile phase for about 30 min.
Injection 10 µL.
Run time 1.5 times the retention time of isoconazole.
Retention time Econazole = about 10 min; isoconazole = about 14 min.
System suitability Reference solution (a):
- — resolution: minimum 5.0 between the peaks due to econazole and isoconazole; if necessary, adjust the composition of the mobile phase.
- — impurities A, B, C: for each impurity, not more than the area of the principal peak in the chromatogram obtained with reference solution (b) (0.25 per cent);
- — total: not more than twice the area of the principal peak in the chromatogram obtained with reference solution (b) (0.5 per cent);
- — disregard limit: 0.2 times the area of the principal peak in the chromatogram obtained with reference solution (b) (0.05 per cent); disregard the peak due to the nitrate ion.
Maximum 0.5 per cent, determined on 1.000 g by drying in an oven at 105 °C for 2 h.
Maximum 0.1 per cent, determined on 1.0 g.
Dissolve 0.350 g in 75 mL of a mixture of 1 volume of anhydrous acetic acid R and 7 volumes of methyl ethyl ketone R. Titrate with 0.1 M perchloric acid, determining the end-point potentiometrically (2.2.20).
1 mL of 0.1 M perchloric acid is equivalent to 47.91 mg of C18H15Cl4N3O4.
Protected from light.
Specified impurities A, B, C.
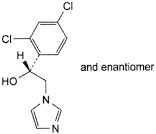
A. (1RS)-1-(2,4-dichlorophenyl)-2-(1H-imidazol-1-yl)ethanol,
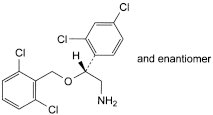
B. (2RS)-2-[(2,6-dichlorobenzyl)oxy]-2-(2,4-dichlorophenyl)ethanamine,
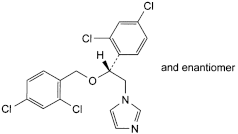
C. 1-[(2RS)-2-[(2,4-dichlorobenzyl)oxy]-2-(2,4-dichlorophenyl)ethyl]-1H-imidazole.
Ph Eur