- British Pharmacopoeia Volume III
- Formulated Preparations: Specific Monographs
Hydrochlorothiazide Tablets |
Thiazide diuretic.
Hydrochlorothiazide Tablets contain Hydrochlorothiazide.
The tablets comply with the requirements stated under Tablets and with the following requirements.
92.5 to 107.5% of the stated amount.
Carry out the method for thin-layer chromatography, Appendix III A, using the following solutions.
(1) Triturate a quantity of the powdered tablets containing 10 mg of Hydrochlorothiazide with 10 mL of acetone and filter.
(2) 0.1% w/v of hydrochlorothiazide BPCRS in acetone.
(a) Use as the coating silica gel GF254.
(b) Use the mobile phase as described below.
(c) Apply 5 µL of each solution.
(d) Develop the plate to 15 cm.
(e) After removal of the plate, dry in a current of air, examine under ultraviolet light (254 nm) and then treat the plate by Method I and examine again.
By each method of visualisation the principal spot in the chromatogram obtained with solution (1) corresponds in colour and intensity to that in the chromatogram obtained with solution (2).
Carry out the method for liquid chromatography, Appendix III D, using the following solutions.
(1) Shake a quantity of the powdered tablets containing 50 mg of Hydrochlorothiazide with 25 mL of a mixture of equal volumes of acetonitrile and methanol and dilute to 100 mL with phosphate buffer solution pH 3.2 R1. Filter through a glass-fibre filter (Whatman 0.45µ GD/X is suitable).
(2) Dilute 1 volume of solution (1) to 100 volumes with a mixture containing 1 volume of methanol, 1 volume of acetonitrile and 2 volumes of phosphate buffer solution pH 3.2 R1.
(3) Dissolve, with the aid of ultrasound, 15 mg each of hydrochlorothiazide BPCRS and chlorothiazide BPCRS in 25 mL of a mixture of equal volumes of acetonitrile and methanol and dilute to 100 mL with phosphate buffer solution pH 3.2 R1. Dilute 5 volumes of this solution to 100 volumes with the same solvent mixture.
(a) Use a stainless steel column (10 cm × 4.6 mm) packed with octadecylsilyl silica gel for chromatography (3 µm) (Phenosphere ODS 3µ or Microsorb ODS 3µ is suitable).
(b) Use gradient elution and the mobile phases described below.
(c) Use a flow rate of 0.8 mL per minute.
(d) Use an ambient column temperature.
(e) Use a detection wavelength of 224 nm.
(f) Inject 20 µL of each solution.
A mixture of 10 volumes of tetrahydrofuran, 60 volumes of methanol and 940 volumes of phosphate buffer solution pH 3.2 R1.
A mixture of 50 volumes of tetrahydrofuran, 500 volumes of methanol and 500 volumes of phosphate buffer solution pH 3.2 R1.
Equilibrate the column for at least 20 minutes with mobile phase A. Inject 20 µL. Carry out a linear gradient elution using the following gradient programme.
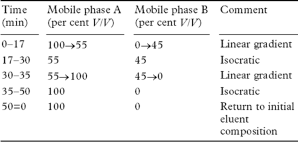
The test is not valid unless, in the chromatogram obtained with solution (3), the resolution factor between the peaks corresponding to chlorothiazide and hydrochlorothiazide is at least 2.5. If necessary, adjust the composition of the mobile phase or the time programme of the linear gradient.
In the chromatogram obtained with solution (1):
the area of any secondary peak is not greater than the area of the principal peak in the chromatogram obtained with solution (2) (1%);
the sum of the areas of any secondary peaks is not greater than 2.5 times the area of the principal peak in the chromatogram obtained with solution (2) (2.5%).
Disregard any peak with an area less than 0.1 times the area of the principal peak in the chromatogram obtained with solution (2) (0.1%).
Weigh and powder 20 tablets. To a quantity of the powder containing 30 mg of Hydrochlorothiazide add 50 mL of 0.1m sodium hydroxide, shake for 20 minutes and dilute to 100 mL with 0.1m sodium hydroxide. Mix, filter, dilute 5 mL of the filtrate to 100 mL with water and measure the absorbance of the resulting solution at the maximum at 273 nm, Appendix II B. Calculate the content of C7H8ClN3O4S2 taking 520 as the value of A(1%, 1 cm) at the maximum at 273 nm.