- British Pharmacopoeia Volume III
- Formulated Preparations: Specific Monographs
Insulin Aspart Injection |
Hormone; treatment of diabetes mellitus.
Insulin Aspart Injection is a sterile, neutral, aqueous solution of Insulin Aspart.
The injection complies with the requirements stated under Insulin Preparations with the modifications described below.
90.0 to 110.0% of the stated amount.
A colourless liquid, free from turbidity and foreign matter; during storage traces of a very fine sediment may be deposited.
In the Assay, the retention time of the principal peak in the chromatogram obtained with solution (1) corresponds to that in the chromatogram obtained with solution (2).
Carry out the method for liquid chromatography, Appendix III D, as described under Assay using the normalisation procedure.
In the chromatogram obtained with solution (1), the area of the peak corresponding to B28isoAsp insulin aspart is not more than 2.5%, the total area of the peaks corresponding to A21Asp insulin aspart, B3Asp insulin aspart and B3isoAsp insulin aspart is not more than 5%, and the total area of other peaks corresponding to impurities is not more than 3.5%.
Not more than 40 µg per 100 units of insulin aspart, determined by atomic absorption spectrometry, Appendix II D Method I.
Test solution Shake the preparation gently and dilute a volume containing 100 units of insulin aspart to 25.0 mL with 0.01m hydrochloric acid. Dilute, if necessary, to a suitable concentration of zinc (for example 0.1 µg to 1.0 µg of Zn per millilitre) with 0.01m hydrochloric acid.
Reference solutions Use solutions containing a suitable range of concentrations, for example 0.20 µg, 0.40 µg, 0.60 µg, 0.80 µg and 1.00 µg of Zn per millilitre, freshly prepared by diluting zinc standard solution (5 mg/mL Zn) with 0.01m hydrochloric acid.
Measure the absorbance at 213.9 nm using a zinc hollow-cathode lamp as source of radiation and an air-acetylene flame of suitable composition (for example 11 litres of air and 2 litres of acetylene per minute).
Carry out the test for bacterial endotoxins, Appendix XIV C. The endotoxin limit concentration is less than 80 IU of endotoxin per 100 units of insulin aspart.
Carry out the method for liquid chromatography, Appendix III D, using the following solutions.
(1) Dilute, if necessary, the preparation being examined with 0.01m hydrochloric acid to produce a solution containing 0.4% w/v insulin aspart. Add 4µL of 6m hydrochloric acid per mL to this solution to obtain a clear acid solution. Maintain the solution at 2° to 8° and use within 48 hours.
(2) Dissolve the contents of a vial of insulin aspart EPCRS in 0.01m hydrochloric acid to produce a solution containing 0.4% w/v insulin aspart. Maintain the solution at 2° to 8° and use within 48 hours.
(3) Use an appropriate solution with a content of B3Asp insulin aspart and A21Asp insulin aspart of not less than 1%. This may be achieved by storing solution (2) at room temperature for 1 to 3 days. Maintain the solution at 2° to 8° and use within 72 hours.
(a) Stainless steel column (25 cm x 4.6 mm) packed with octadecylsilyl silica gel for chromatography (5 µm) (Lichrosorb RP18 is suitable).
(b) Gradient elution using the mobile phases described below.
(c) Flow rate of 1 mL per minute.
(d) Column temperature of 35°.
(e) Detection wavelength of 214 nm.
(f) An injection volume of 10 µL for each solution.
Mobile phase A Dissolve 70 g of anhydrous sodium sulfate in approximately 4500 mL of water; add 6.5 mL of orthophosphoric acid and adjust to pH 3.4, if necessary, with dilute sodium hydroxide solution. Dilute to 5000 mL with water; filter and degas. Mix 9 volumes of the solution with 1 volume of acetonitrile for chromatography.
Mobile phase B Mix equal volumes of water and acetonitrile for chromatography.
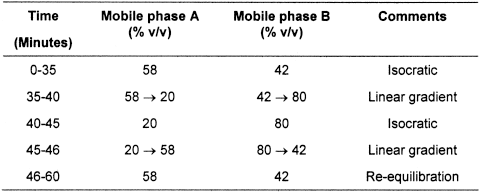
When the chromatograms are recorded under the prescribed conditions the relative retention times with reference to insulin aspart (retention time, 20 to 26 minutes) are: B28isoAsp insulin aspart, approximately 0.9; B3Asp insulin aspart plus A21Asp insulin aspart (generally co eluted), approximately 1.3; B3isoAsp insulin aspart, approximately 1.5.
The test is not valid unless, in the chromatogram obtained with solution (3), the resolution factor between the peak due to insulin aspart and the peak due to A21Asp insulin aspart plus B3Asp insulin aspart is greater than 1.6.
The test is not valid unless the symmetry factor of the principal peak in the chromatogram obtained with solution (2) is less than 1.8.
Calculate the content of insulin aspart C256H381N65O79S6, together with the content of B28isoAsp insulin aspart, A21Asp insulin aspart, B3Asp insulin aspart and B3isoAsp insulin aspart using the areas of the corresponding peaks in the chromatograms obtained with solution (1) and solution (2) and the declared content of insulin aspart together with the content of B28isoAsp insulin aspart, A21Asp insulin aspart, B3Asp insulin aspart and B3isoAsp insulin aspart(1) in insulin aspart EPCRS.
The label states the potency in units per mL.
1 100 units are equivalent to 3.50 mg of insulin aspart.