- British Pharmacopoeia Volume III
- Formulated Preparations: Specific Monographs
Mesalazine Enema |
Aminosalicylate; treatment of ulcerative colitis.
Mesalazine Enema is a rectal suspension containing Mesalazine in a suitable vehicle.
The enema complies with the requirements stated under Rectal Preparations and with the following requirements.
95.0 to 105.0% of the stated amount.
Filter a quantity of the enema containing 1.0 g of Mesalazine and discard the filtrate. Dry the residue at 110°. The infrared spectrum of the residue, Appendix II A, is concordant with the reference spectrum of mesalazine (RS 454).
Carry out the method for liquid chromatography, Appendix III D, using the following solutions prepared immediately before use.
(1) Mix for 10 minutes with the aid of ultrasound, a quantity of the enema containing 1 g of Mesalazine in 700 mL of 0.01m hydrochloric acid, add sufficient 0.01m hydrochloric acid to produce 1 L, mix and filter through a 0.45-µm membrane filter.
(2) Dilute 1 volume of solution (1) to 100 volumes with 0.01m hydrochloric acid and further dilute 1 volume of the resulting solution to 10 volumes with 0.01m hydrochloric acid.
(3) Dilute 1 volume of a 0.01% w/v solution of 3-aminosalicylic acid in 0.01m hydrochloric acid to 100 volumes with solution (1).
(4) 0.0001% w/v each of 4-aminosalicylic acid, 2,5-dihydroxybenzoic acid, 2-chlorobenzoic acid, 2-chloro-5-nitrobenzoic acid, 5-nitrosalicylic acid, sulfanilic acid, 3-nitrosalicylic acid and 0.0003% w/v of salicylic acid in 0.01m hydrochloric acid.
(5) 0.01% w/v of mesalazine for peak identification EPCRS in 0.01m hydrochloric acid.
(6) Dilute 3 volumes of solution (2) to 10 volumes with 0.01m hydrochloric acid.
(a) Use a stainless steel column (25 cm × 4.6 mm) packed with end-capped octadecylsilyl silica gel for chromatography (5 µm) (XTerra MS C18 is suitable).
(b) Use gradient elution and the mobile phase described below.
(c) Use a flow rate of 1 mL per minute.
(d) Use a column temperature of 40°.
(e) Use a detection wavelength of 240 nm.
(f) Inject 20 µL of each solution.
Mobile phase A A 0.69% w/v solution of sodium dihydrogen phosphate monohydrate, adjusted to pH 6.2 with dilute sodium hydroxide.
Mobile phase B 40 volumes of acetonitrile and 60 volumes of mobile phase A.
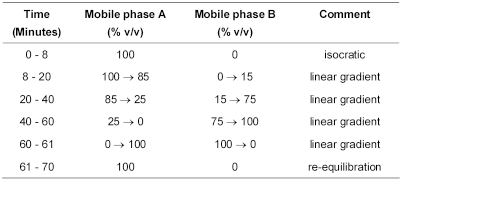
Use the chromatogram obtained with solution (3) to identify the peak due to impurity F; use the chromatogram obtained with solution (4) to identify the peaks due to impurities E, G, H, L, M and R; use the chromatogram supplied with mesalazine for peak identification EPCRS and the chromatogram obtained with solution (5) to identify the peaks due to impurities J and P.
When the chromatograms are recorded under the prescribed conditions, the relative retentions with reference to mesalazine (retention time = about 6 minutes) are: impurity O = about 0.55; impurity J = about 0.6; impurity E = about 0.8; impurity F = about 1.36; impurity G = about 1.4; impurity P = about 1.5; impurity L = about 2.0; impurity M = about 3.3; impurity H = about 3.5; impurity R = about 5.1; impurity N = about 5.5.
In the chromatogram obtained with solution (3):
the peak-to-valley ratio is at least 3.0, where Hp is the height above the baseline of the peak due to 3-aminosalicylic acid and Hv is the height above the baseline of the lowest point of the curve separating this peak from the peak due to mesalazine.
Identify any peaks in the chromatogram obtained with solution (1) corresponding to impurities E, G, H, J, L, M, O, P and R using solutions (4) and (5) and multiply the area of these peaks by the corresponding correction factors: impurity E, 1.3; impurity G, 1.4; impurity H, 1.4; impurity J, 2.0; impurity L, 4.5; impurity M, 1.7; impurity O, 0.6; impurity P, 0.6; impurity R. 1.3.
In the chromatogram obtained with solution (1):
the area of any peak corresponding to impurity H is not greater than 3 times the area of the principal peak in the chromatogram obtained with solution (2) (0.3%);
the area of any peak corresponding to impurity E, F, G, J, L, M, P or R is not greater than 1.5 times the area of the principal peak in the chromatogram obtained with solution (2) (0.15%);
the area of any other secondary peak is not greater than the area of the principal peak in the chromatogram obtained with solution (2) (0.1%);
the sum of the areas of any secondary peaks is not greater than 5 times the area of the principal peak in the chromatogram obtained with solution (2) (0.5 %).
Disregard any peak with an area less than the area of the principal peak in the chromatogram obtained with solution (6) (0.03%).
Carry out the method for liquid chromatography, Appendix III D, using the following solutions and freshly prepared mobile phases.
(1) Mix for 10 minutes with the aid of ultrasound, a quantity of the enema containing 1 g of Mesalazine in 400 mL of mobile phase A with occasional shaking, add sufficient mobile phase A to produce 1 L, mix and filter through a 0.45-µm membrane filter.
(2) 0.00002% w/v of 2-aminophenol in mobile phase A.
(3) To 1 volume of solution (1) add sufficient of mobile phase A to produce 200 volumes, mix 1 volume of this solution with 1 volume of 0.0005% w/v of 2-aminophenol in mobile phase A.
(a) Use a stainless steel column (25 cm × 4.6 mm) packed with spherical end-capped octadecylsilyl silica gel for chromatography (3 µm) (Nucleosil C18e is suitable).
(b) Use gradient elution and the mobile phases described below.
(c) Use a flow rate of 1 mL per minute.
(d) Use an ambient column temperature.
(e) Use a detection wavelength of 220 nm.
(f) Inject 20 µL of each solution.
When the chromatograms are recorded under the prescribed conditions, the relative retention with reference to mesalazine (retention time = about 9 minutes) for 2-aminophenol is about 0.9.
Mobile phase A 0.22% w/v of perchloric acid and 0.1% w/v of phosphoric acid.
Mobile phase B 0.17% w/v of perchloric acid and 0.1% w/v of phosphoric acid in acetonitrile R1.
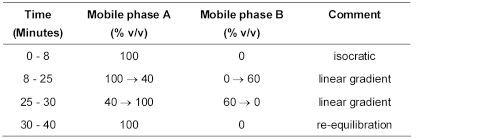
The test is not valid unless, in the chromatogram obtained with solution (3), the resolution factor between the two principal peaks is at least 3.0.
In the chromatogram obtained with solution (1):
the area of any peak corresponding to 2-aminophenol is not greater than the area of any corresponding peak in the chromatogram obtained with solution (2) (200 ppm).
Carry out the method for liquid chromatography, Appendix III D, using the following solutions.
(1) Add 5 drops of 1m sodium hydroxide to a quantity of the enema containing 50 mg of Mesalazine, add 15 mL of the mobile phase, mix for 20 minutes with the aid of ultrasound, add sufficient of the mobile phase to produce 25 mL and filter through a 0.45-µm membrane filter.
(2) 0.00000278% w/v of aniline hydrochloride in the mobile phase.
(a) Use a stainless steel column (25 cm × 4 mm) packed with end-capped octadecylsilyl silica gel for chromatography (5 µm) (Lichrospher RP18e is suitable).
(b) Use isocratic elution and the mobile phase described below.
(c) Use a flow rate of 1 mL per minute.
(d) Use a column temperature of 40°.
(e) Use a detection wavelength of 205 nm.
(f) Inject 50 µL of each solution.
The retention time of aniline is about 15 minutes.
15 volumes of methanol and 85 volumes of a solution containing 0.141% w/v of potassium dihydrogen orthophosphate and 0.047% w/v of disodium hydrogen orthophosphate dihydrate previously adjusted to pH 8.0 with 4.2% w/v of sodium hydroxide.
In the chromatogram obtained with solution (1):
the area of any peak corresponding to aniline is not greater than the area of any corresponding peak in the chromatogram obtained with solution (2) (10 ppm).
Carry out the method for liquid chromatography, Appendix III D, using the following solutions.
(1) Mix a quantity of the enema containing 25 mg of Mesalazine in 15 mL of 0.1m hydrochloric acid for 50 minutes with the aid of ultrasound, with additional vortex mixing at 10 minute intervals; add sufficient 0.1m hydrochloric acid to produce 25 mL, mix and filter. To 1 volume of the filtrate add sufficient 0.1m hydrochloric acid to produce 50 volumes.
(2) 0.002% w/v of mesalazine BPCRS prepared by dissolving in 0.1m hydrochloric acid with the aid of ultrasound.
(3) Prepare a 0.01% w/v of 3-aminosalicylic acid in 0.1m hydrochloric acid and dilute 1 volume of this solution to 100 volumes with a 0.1% w/v solution of mesalazine BPCRS in 0.1m hydrochloric acid.
(a) Use a stainless steel column (25 cm × 4.6 mm) packed with end-capped octadecylsilyl silica gel for chromatography (5 µm) (XTerra MS C18 is suitable).
(b) Use isocratic elution and the mobile phase described below.
(c) Use a flow rate of 1 mL per minute.
(d) Use a column temperature 40°.
(e) Use a detection wavelength of 240 nm.
(f) Inject 20 µL of each solution.
A 0.69% w/v solution of sodium dihydrogen phosphate monohydrate, adjusted to pH 6.2 with dilute sodium hydroxide solution.
In the chromatogram obtained with solution (3):
the peak-to-valley ratio is at least 3.0, where Hp is the height above the baseline of the peak due to 3-aminosalicylic acid and Hv is the height above the baseline of the lowest point of the curve separating this peak from the peak due to mesalazine.
determination of content
Calculate the content of C7H7NO3 in the enema using the declared content of C7H7NO3 in mesalazine BPCRS.
The label indicates the pharmaceutical form as 'rectal suspension'.
The impurities limited by the requirements of this monograph include those listed under Mesalazine.