- British Pharmacopoeia Volume IV
- Appendices
Appendix V B. Determination of Freezing Point |
The freezing point is the maximum temperature occurring during the solidification of a supercooled liquid.
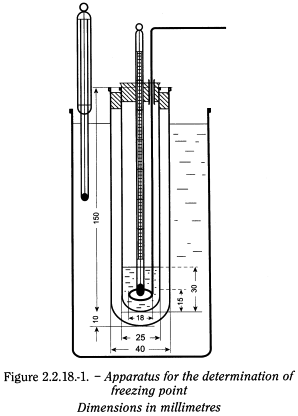
Apparatus The apparatus (see Figure 2.2.18.-1) consists of a test-tube about 25 mm in diameter and 150 mm long placed inside a test-tube about 40 mm in diameter and 160 mm long. The inner tube is closed by a stopper which carries a thermometer about 175 mm long and graduated in 0.2 °C fixed so that the bulb is about 15 mm above the bottom of the tube. The stopper has a hole allowing the passage of the stem of a stirrer made from a glass rod or other suitable material formed at one end into a loop of about 18 mm overall diameter at right angles to the rod. The inner tube with its jacket is supported centrally in a 1 litre beaker containing a suitable cooling liquid to within 20 mm of the top. A thermometer is supported in the cooling bath.
Method Place in the inner tube sufficient quantity of the liquid or previously melted substance to be examined, to cover the thermometer bulb and determine the approximate freezing point by cooling rapidly. Place the inner tube in a bath about 5 °C above the approximate freezing point until all but the last traces of crystals are melted. Fill the beaker with water or a saturated solution of sodium chloride, at a temperature about 5 °C lower than the expected freezing point, insert the inner tube into the outer tube, ensuring that some seed crystals are present, and stir thoroughly until solidification takes place. Note the highest temperature observed during solidification.