- British Pharmacopoeia Volume IV
- Appendices
Appendix XI S. Determination of Aflatoxin B1 in Herbal Drugs |
CAUTION: aflatoxins are very toxic and carcinogenic. Perform manipulations under an extraction hood whenever possible. Take particular precautions, such as use of a glove box, when toxins are in dry form because of their electrostatic properties and the tendency to disperse through the working areas. Decontamination procedures for laboratory wastes of aflatoxins were developed by the International Agency for Research on Cancer (IARC).
Aflatoxins are naturally occurring mycotoxins produced mainly by Aspergillus flavus and Aspergillus parasiticus. These fungi are common and widespread in nature and are most often found when certain grains are grown under conditions of stress such as drought. The mould occurs in soil, decaying vegetation, hay, and grains undergoing microbial spoilage, and invades all types of organic substrates whenever and wherever the conditions are favourable for its growth. Favourable conditions include high moisture content and high temperature. At least 13 different types of aflatoxin are produced in nature and most of these are known to be highly toxic and carcinogenic. Aflatoxin B1 is considered the most toxic. Herbal drugs that are subject to contamination by aflatoxins are tested by a validated method.
Unless otherwise indicated in the monograph, herbal drugs contain not more than 2 µg/kg of aflatoxin B1. The competent authority may also require compliance with a limit of 4 µg/kg for the sum of aflatoxins B1, B2, G1 and G2.
The method described below is cited as an example of a method that has been shown to be suitable for devil's claw root, ginger and senna pods. Its suitability for other herbal drugs must be demonstrated or another validated method used.
Liquid chromatography (2.2.29).
Aflatoxins are subject to light degradation. Carry out the determination protected from daylight by using UV-absorbing foil on windows in combination with subdued light, or curtains or blinds in combination with artificial light (fluorescent tubes are acceptable). Protect aflatoxin-containing solutions from daylight.
Rinse glassware before use with a 10 per cent V/V solution of sulphuric acid R and then rinse carefully with distilled water R until no more acid is present.
Test solution Use an immunoaffinity column containing antibodies against aflatoxin B1 with a capacity of not less than 100 ng of aflatoxin B1 and which gives a recovery of not less than 80 per cent when a solution of 5 ng of aflatoxin B1 in a mixture of 12.5 ml of methanol R and 87.5 ml of water R is passed through. Allow the immunoaffinity column to reach room temperature. To 5.00 g of the powdered drug (500) (2.9.12) add 100 ml of a mixture of 30 volumes of water R and 70 volumes of methanol R and extract by sonication for 30 min. Filter through folded filter paper. Pipette 10.0 ml of the clear filtrate into a 150 ml conical flask. Add 70 ml of water R. Pass 40 ml through the immunoaffinity column at a flow rate of 3 ml/min (not exceeding 5 ml/min). Wash the column with 2 volumes, each of 10 ml, of water R at a flow rate not exceeding 5 ml/min and dry by applying a slight vacuum for 5-10 s or by passing air through the immunoaffinity column by means of a syringe for 10 s. Apply 0.5 ml of methanol R to the column and allow to pass through by gravity. Collect the eluate in a 5 ml volumetric flask. After 1 min, apply a 2nd portion of 0.5 ml of methanol R. After a further 1 min, apply a 3rd portion of 0.5 ml of methanol R. Collect most of the applied elution solvent by pressing air through or applying vacuum to the column. Dilute to 5 ml with water R and shake well. If the solution is clear it can be used directly for analysis. Otherwise, pass it through a disposable filter unit prior to injection. Use a disposable filter unit (e.g. 0.45 µm pore size polytetrafluoroethylene filter) that has been shown not to cause loss of aflatoxin by retention.
Aflatoxin B1 primary stock solution Dissolve aflatoxin B1 R in a mixture of 2 volumes of acetonitrile R and 98 volumes of toluene R to give a 10 µg/ml solution. To determine the exact concentration of aflatoxin B1 in the stock solution, record the absorption curve (2.2.25) between 330 nm and 370 nm in quartz cells.
Calculate the aflatoxin B1 mass concentration, in micrograms per millilitre, using the following expression:
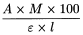
A |
= |
absorbance determined at the maximum of the absorption curve; |
M |
= |
molar mass of aflatoxin B1 (312 g/mol); |
∊ |
= |
molar absorptivity of aflatoxin B1 in the toluene-acetonitrile mixture (1930 m2/mol); |
l |
= |
optical path length of the cell (1 cm). |
Aflatoxin B1 secondary stock solution Prepare a secondary stock solution containing 100 ng/ml aflatoxin B1 by diluting aflatoxin B1 primary stock solution with a mixture of 2 volumes of acetonitrile R and 98 volumes of toluene R. Wrap the flask tightly in aluminium foil and store it below 4 °C. Before use, do not remove the aluminium foil until the contents have reached room temperature. If the solution has to be stored for a long period (for example, 1 month), weigh the flask and record the mass before and after each use of the solution.
Aflatoxin B1 standard solutions Place the volumes of aflatoxin secondary stock solution indicated in Table 2.8.18.-1 in separate 250 ml volumetric flasks. Pass a stream of nitrogen through at room temperature until the solvent has just evaporated. To each flask, add 75 ml of methanol R, allow the aflatoxin B1 to dissolve and dilute to 250 ml with water R.
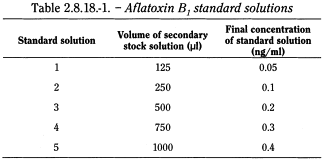
Calibration curve Prepare the calibration curve using aflatoxin B1 standard solutions 1 to 5, which cover a range equivalent to 1-8 µg/kg of aflatoxin B1 in the herbal drug. Check the plot for linearity. If the content of aflatoxin B1 in the sample to be examined is outside of the calibration range, the test solution must be diluted to an aflatoxin content that is appropriate for the established calibration curve.
Column:
- — size: l = 0.25 m, Ø = 4.6 mm;
- — stationary phase: octadecylsilyl silica gel for chromatography R (5 µm).
Mobile phase:
- — mobile phase A (for post-column derivatisation with photochemical reactor or pyridinium bromide): acetonitrile R, methanol R, water R (2:3:6 V/V/V);
- — mobile phase B (for post-column derivatisation with electrochemically derived bromine): add 0.12 g of potassium bromide R and 350 µl of dilute nitric acid R1 per litre of mobile phase A.
Flow rate 1 ml/min.
Detection: Fluorescence detector with a 360 nm excitation filter and a 420 nm cut-off emission filter, or equivalent. Recommended settings for adjustable detectors are 365 nm (excitation wavelength) and 435 nm (emission wavelength).
Injection: 500 µl.
Post-column derivatisation with pyridinium hydrobromide perbromide (PBPB):
- — pulseless pump;
- — T-piece with zero dead volume;
- — polytetrafluoroethylene reaction tube, l = 0.45 m, Ø = 0.5 mm;
- — mobile phase A;
- — post-column derivatisation reagent: dissolve 50 mg of pyridinium hydrobromide perbromide R in 1000 ml of water R (store protected from light and use within 4 days);
- — flow rate of the derivatisation reagent: 0.4 ml/min.
Post-column derivatisation with photochemical reactor (PHRED)
- — reactor unit with one 254 nm low pressure mercury UV bulb (minimum 8 W);
- — polished support plate;
- — knitted reactor coil: polytetrafluoroethylene tubing knitted tightly around the UV bulb, l = 25 m, Ø = 0.25 mm, nominal void volume 1.25 ml;
- — exposure time: 2 min;
- — mobile phase A.
Post-column derivatisation with electrochemically generated bromine (KOBRA):
- — KOBRA-cell: electrochemical cell that generates a reactive form of bromine for derivatisation of aflatoxins, resulting in enhanced fluorescence; available from various commercial suppliers;
- — Derivation direct-current supply in series with the KOBRA-cell, providing a constant current of about 100 µA;
- — polytetrafluoroethylene reaction tube, l = 0.12 m, Ø = 0.25 mm;
- — mobile phase B.
Elution order aflatoxin G2, aflatoxin G1, aflatoxin B2, aflatoxin B1.
Calculation calculate the calibration curve y = ax + b, with aflatoxin B1 concentration (ng/ml) on the x-axis and the signal (S) on the y-axis. The aflatoxin B1 concentration (C) in a measured solution is equal to 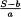
Calculate the aflatoxin B1 content of the herbal drug, in nanograms per gram, using the following expression:
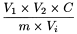
m |
= |
mass of the herbal drug taken for analysis, in grams; |
V1 |
= |
volume of the solvent used for extraction, in millilitres; |
Vi |
= |
aliquot taken for immunoaffinity clean-up, in millilitres; |
V2 |
= |
final volume of solution after elution from the immunoaffinity column and dilution, in millilitres; |
C |
= |
measured aflatoxin B1 concentration of the test solution, in nanograms per millilitre. |
The presence of aflatoxin B1 may be confirmed by recording the chromatogram without post-column derivatisation, which leads to a large decrease (greater than 10-fold) in the response due to aflatoxin B1.