- British Pharmacopoeia Volume III
- Blood-related Products
Albumin Solution |
 |
Albumin; Human Albumin
(Human Albumin Solution, Ph Eur monograph 0255)
Ph Eur
Human albumin solution is an aqueous solution of protein obtained from plasma that complies with the requirements of the monograph on Plasma for fractionation, human (0853).
Separation of the albumin is carried out under controlled conditions, particularly of pH, ionic strength and temperature so that in the final product not less than 95 per cent of the total protein is albumin. Human albumin solution is prepared as a concentrated solution containing 150-250 g/l of total protein or as an isotonic solution containing 35-50 g/l of total protein. A suitable stabiliser against the effects of heat, such as sodium caprylate (sodium octanoate) or N-acetyltryptophan or a combination of these 2, at a suitable concentration, may be added but no antimicrobial preservative is added at any stage during preparation. The solution is passed through a bacteria-retentive filter and distributed aseptically into sterile containers which are then closed so as to prevent contamination. The solution in its final container is heated to 60 ± 1.0 °C and maintained at this temperature for not less than 10 h. The containers are then incubated at 30-32 °C for not less than 14 days or at 20-25 °C for not less than 4 weeks and examined visually for evidence of microbial contamination.
A clear, slightly viscous liquid; it is almost colourless, yellow, amber or green.
Examine by a suitable immunoelectrophoresis technique. Using antiserum to normal human serum, compare normal human serum and the preparation to be examined, both diluted to contain 10 g/l of protein. The main component of the preparation to be examined corresponds to the main component of normal human serum. The preparation may show the presence of small quantities of other plasma proteins.
6.7 to 7.3.
Dilute the preparation to be examined with a 9 g/l solution of sodium chloride R to obtain a solution containing 10 g/l of protein.
Dilute the preparation to be examined with a 9 g/l solution of sodium chloride R to obtain a solution containing about 15 mg of protein in 2 ml. To 2.0 ml of this solution in a round-bottomed centrifuge tube add 2 ml of a 75 g/l solution of sodium molybdate R and 2 ml of a mixture of 1 volume of nitrogen-free sulphuric acid R and 30 volumes of water R. Shake, centrifuge for 5 min, decant the supernatant liquid and allow the inverted tube to drain on filter paper. Determine the nitrogen in the residue by the method of sulphuric acid digestion (2.5.9) and calculate the quantity of protein by multiplying by 6.25. The preparation contains not less than 95 per cent and not more than 105 per cent of the quantity of protein stated on the label.
Zone electrophoresis (2.2.31).
Use strips of suitable cellulose acetate gel or agarose gel as the supporting medium and barbital buffer solution pH 8.6 R1 as the electrolyte solution.
If cellulose acetate is the supporting material, the method described below can be used. If agarose gels are used, and because they are normally part of an automated system, the manufacturer's instructions are followed instead.
Test solution Dilute the preparation to be examined with a 9 g/l solution of sodium chloride R to a protein concentration of 20 g/l.
Reference solution Dilute human albumin for electrophoresis BRP with a 9 g/l solution of sodium chloride R to a protein concentration of 20 g/l.
To a strip apply 2.5 µl of the test solution as a 10 mm band or apply 0.25 µl per millimetre if a narrower strip is used. To another strip, apply in the same manner the same volume of the reference solution. Apply a suitable electric field such that the most rapid band migrates at least 30 mm. Treat the strips with amido black 10B solution R for 5 min. Decolorise with a mixture of 10 volumes of glacial acetic acid R and 90 volumes of methanol R until the background is just free of colour. Develop the transparency of the strips with a mixture of 19 volumes of glacial acetic acid R and 81 volumes of methanol R. Measure the absorbance of the bands at 600 nm in an instrument having a linear response over the range of measurement. Calculate the result as the mean of 3 measurements of each strip.
System suitability In the electropherogram obtained with the reference solution on cellulose acetate or on agarose gels, the proportion of protein in the principal band is within the limits stated in the leaflet accompanying the reference preparation.
Results In the electropherogram obtained with the test solution on cellulose acetate or on agarose gels, not more than 5 per cent of the protein has a mobility different from that of the principal band.
Liquid chromatography (2.2.29).
Test solution Dilute the preparation to be examined with a 9 g/l solution of sodium chloride R to a concentration suitable for the chromatographic system used. A concentration in the range of 4-12 g/l and injection of 50-600 µg of protein are usually suitable.
- — size: l = 0.6 m, Ø = 7.5 mm, or l = 0.3 m, Ø = 7.8 mm;
- — stationary phase: hydrophilic silica gel for chromatography R, of a grade suitable for fractionation of globular proteins with relative molecular masses in the range 10 000 to 500 000.
Mobile phase Dissolve 4.873 g of disodium hydrogen phosphate dihydrate R, 1.741 g of sodium dihydrogen phosphate monohydrate R, 11.688 g of sodium chloride R and 50 mg of sodium azide R in 1 litre of water R.
Flow rate 0.5 ml/min.
Detection Spectrophotometer at 280 nm.
The peak due to polymers and aggregates is located in the part of the chromatogram representing the void volume. Disregard the peak due to the stabiliser. The area of the peak due to polymers and aggregates is not greater than 10 per cent of the total area of the chromatogram (corresponding to about 5 per cent of polymers and aggregates).
Dilute the preparation to be examined using a 9 g/l solution of sodium chloride R to obtain a solution containing 10 g/l of protein. The absorbance (2.2.25) of the solution measured at 403 nm using water R as the compensation liquid is not greater than 0.15.
Maximum 35 IU/ml.
Maximum 2.0 × 102 µg/l.
Atomic absorption spectrometry (2.2.23, Method I or II).
Use a furnace as atomic generator.
Use plastic containers for preparation of the solutions and use plastic equipment where possible. Wash glassware (or equipment) in nitric acid (200 g/l HNO3) before use.
Test solution Use the preparation to be examined, diluted if necessary.
Reference solutions Prepare at least 3 reference solutions in a range spanning the expected aluminium concentration of the preparation to be examined, for example by diluting aluminium standard solution (10 ppm Al) R with a 1 g/l solution of octoxinol 10 R.
Monitor solution Add aluminium standard solution (10 ppm Al) R or a suitable certified reference material to the test solution in a sufficient amount to increase the aluminium concentration by 20 µg/l.
Blank solution 1 g/l solution of octoxinol 10 R.
Wavelength 309.3 nm or other suitable wavelength.
Slit width 0.5 nm.
Tube Pyrolytically coated, with integrated platform.
Background corrector Off.
Atomisation device Furnace; fire between readings.
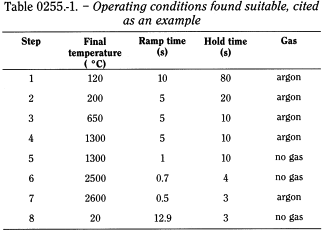
The operating conditions in Table 0255.-1 are cited as an example of conditions found suitable for a given apparatus; they may be modified to obtain optimum conditions.
Injection Each of the following solutions 3 times: blank solution, reference solutions, test solution and monitor solution.
- — the recovery of aluminium added in preparation of the monitor solution is within the range 80-120 per cent.
Prepare a calibration curve from the mean of the readings obtained with the reference solutions and determine the aluminium content of the preparation to be examined using the calibration curve.
Maximum 0.05 mmol of K per gram of protein.
Atomic emission spectrometry (2.2.22, Method I).
Wavelength 766.5 nm.
Maximum 1.60 × 102 mmol/l and not less than 95 per cent and not more than 105 per cent of the content of Na stated on the label.
Atomic emission spectrometry (2.2.22, Method I).
Wavelength 589 nm.
It complies with the test for sterility.
It complies with the test for pyrogens. For a solution containing 35-50 g/l of protein, inject per kilogram of the rabbit's mass 10 ml of the preparation to be examined. For a solution containing 150-250 g/l of protein, inject per kilogram of the rabbit's mass 5 ml of the preparation to be examined.
Protected from light.
The label states:
- — the name of the preparation;
- — the volume of the preparation;
- — the content of protein expressed in grams per litre;
- — the content of sodium expressed in millimoles per litre;
- — that the product is not to be used if it is cloudy or if a deposit has formed;
- — the name and concentration of any added substance (for example stabiliser).
Ph Eur