Stevens Rearrangement
T. S. Stevens et al., J. Chem. Soc. 1928, 3193; 1930, 2107, 2119; 1932, 55, 1926, 1932.
Migration of an alkyl group from a sulfonium or quaternary ammonium salt to an adjacent carbanionic center on treatment with strong base. The product is a rearranged tertiary amine or sulfide:
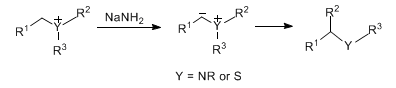
Early reviews: H. E. Zimmerman in Molecular Rearrangements Part 1, P. de Mayo, Ed. (Wiley-Interscience, New York, 1963) pp 345-406; D. J. Cram, Fundamentals of Carbanion Chemistry (Academic Press, New York, 1965) pp 223-229; S. M. Pine, Org. React. 18, 403-464 (1970). Selectivity studies vs Sommelet-Hauser rearrangement, q.v.: T. Kitano et al., J. Chem. Soc. Perkin Trans. 1 1992, 2851; T. Tanaka et al., J. Org. Chem. 57, 5034 (1992). Review: I. E. Markó, Comp. Org. Syn. 3, 913-932 (1991). Comprehensive review and applications in natural product synthesis: J. A. Vanecko et al., Tetrahedron 62, 1043-1062 (2006). Cf. Meisenheimer Rearrangements; [1,2]-Wittig Rearrangement.