Sharpless Epoxidation
T. Katsuki, K. B. Sharpless, J. Am. Chem. Soc. 102, 5974 (1980).
Titanium-catalyzed asymmetric epoxidation of allylic alcohols employing titanium alkoxide, an optically active tartrate ester and an alkyl hydroperoxide. A high degree of enantiomeric purity is attainable having predictable absolute stereochemistry:
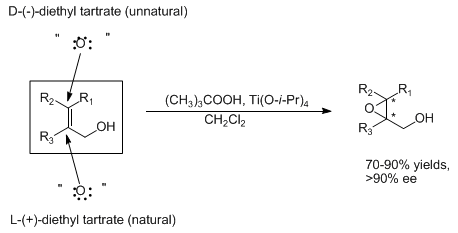
Note: The asterisk at a chiral center denotes a preponderance of either the R or S configuration.
Mechanistic studies: S. S. Woodward et al., J. Am. Chem. Soc. 113, 106 (1991); M. G. Finn, K. B. Sharpless, ibid. 113. Methods development for the synthesis of enantiopure allylic alcohols: D. C. Dittmer et al., J. Org. Chem. 58, 718 (1993). Alkenylsilanols as substrates: T. H. Chan et al., Can. J. Chem. 71, 60 (1993). Reviews: R. A. Johnson, K. B. Sharpless, Comp. Org. Syn. 7, 389-436 (1991); E. Höft, Top. Curr. Chem. 164, 63-77 (1993).