Schmidt Reaction
R. F. Schmidt, Ber. 57, 704 (1924).
Acid-catalyzed addition of hydrazoic acid to carboxylic acids, aldehydes and ketones to give amines, nitriles and amides, respectively. Tertiary alcohols and substituted alkenes yield imines upon treatment with hydrazoic acid:
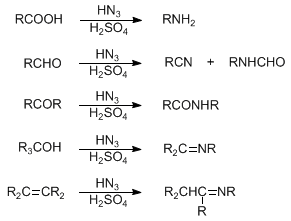
Early reviews: H. Wolff, Org. React. 3, 307-336 (1946); P. A. S. Smith in Molecular Rearrangements Part 1, P. de Mayo, Ed. (Wiley-Interscience, New York, 1963) pp 507-558; D. V. Banthorpe, The Chemistry of the Azido Group, S. Patai, Ed. (Interscience, New York, 1971) pp 405-421; G. I. Koldobskii, Russ. Chem. Rev. 47, 1084 (1978). Application to cyclic ketones: A. Lévai et al., Heterocycles 34, 1523 (1992); J.-Y. Mérour et al., J. Heterocycl. Chem. 31, 87 (1994); to alcohols and alkenes: W. H. Pearson et al., J. Am. Chem. Soc. 115, 10183 (1993). Extension to dialkyl acylphosphonates: M. Sprecher, D. Kost, ibid. 116, 1016 (1994). Review: T. Shioiri, Comp. Org. Syn. 6, 817-821 (1991). Cf. Beckmann Rearrangement; Curtius Rearrangement; Hofmann Reaction; Lossen Rearrangement.