Noyori Hydrogenation
T. Ikariya et al., Chem. Commun. 1985, 922; R. Noyori et al., J. Am. Chem. Soc. 108, 7117 (1986).
Homogeneous asymmetric catalytic hydrogenation of olefinic and carbonyl bonds mediated by enantiopure ruthenium(II) BINAP complexes. The substrates must have coordinating functionalities in neighboring positions which serve as directing groups during the transformation:
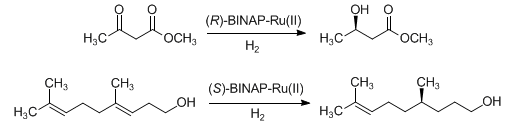
Detailed experimental procedure: M. Kitamura et al., Org. Synth. 71, 1 (1993). Methods development for enamide substrates: idem et al., J. Org. Chem. 59, 297 (1994); E. Vedejs et al., ibid. 64, 6724 (1999). Development and use of arene substituted BINAP catalysts: K. Mashima et al., ibid. 59, 3064 (1994). Reviews: H. Takaya et al. in Adv. Chem. Ser. 230, entitled “Homogeneous Transition Metal Catalyzed Reactions” (ACS, Washington DC, 1992) pp 123-142; R. Noyori, Asymmetric Catalysis in Organic Synthesis (John Wiley & Sons, New York, 1994) pp 16-94.