Knoevenagel Condensation; Doebner Modification
E. Knoevenagel Ber. 31, 2596 (1898); O. Doebner, Ber. 33, 2140 (1900).
Condensation of aldehydes or ketones with active methylene compounds in the presence of ammonia or amines; the use of malonic acid and pyridine is known as the Doebner modification:
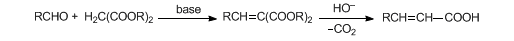
Early reviews: J. R. Johnson, Org. React. 1, 210 (1942); G. Jones, ibid. 15, 204 (1967); H. O. House, Modern Synthetic Reactions (W. A. Benjamin, Menlo Park, California, 2nd ed., 1972) pp 646-653. Development of enantioselective methods: L. F. Tietze, P. Saling, Chirality 5, 329 (1993). Application to the synthesis of indole alkaloids: L. F. Tietze et al., Synthesis 1994, 1185. Modified conditions: J. McNulty et al., Tetrahedron Lett. 39, 8013 (1998); B. M. Choudary et al., J. Mol. Catal. A 142, 361 (1991). Synthetic applications: B. T. Watson, G. E. Christiansen, Tetrahedron Lett. 39, 6087 (1998); R. W. Draper et al., Tetrahedron 56, 1811 (2000). Review: L. F. Tietze, U. Beifuss, Comp. Org. Syn. 2, 341-394 (1991). Cf. Aldol Reaction; Henry Reaction; Ivanov Reaction.