Kiliani-Fischer Synthesis
H. Kiliani, Ber. 18, 3066 (1885); E. Fischer, ibid. 22, 2204 (1889).
Extension of the carbon atom chain of aldoses by treatment with cyanide. Hydrolysis of the cyanohydrins followed by reduction of the lactone yields the homologous aldose:
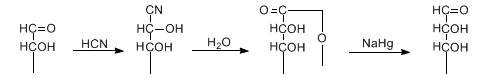
Reviews: C. S. Hudson, Adv. Carbohydr. Chem. 1, 2 (1945); T. Moury, Chem. Rev. 42, 239 (1948); L. Hough, A. C. Richardson, The Carbohydrates 1A, 118 (1972); R. Kuhn, P. Klesse, Ber. 91, 1989 (1958); R. Varma, D. French, Carbohydr. Res. 25, 71 (1972); R. Blazer, T. W. Whalen, J. Am. Chem. Soc. 102, 5082 (1980). Mechanistic study: A. S. Serianni et al., J. Org. Chem. 45, 3329 (1980). Modified conditions: N. Adjé et al., Tetrahedron Lett. 37, 5893 (1996). Stereoselective synthesis: J. Roos, F. Effenberger, Tetrahedron: Asymmetry 10, 2817 (1999). Cf. Urech Cyanohydrin Method.