Julia Olefination (Julia-Lythgoe Olefination)
M. Julia, M.-M. Paris, Tetrahedron Lett. 1973, 4833.
The formation of predominantly trans-olefins via the addition of phenyl sulfones to aldehydes or ketones, followed by alcohol functionalization and subsequent reductive elimination with sodium amalgam:
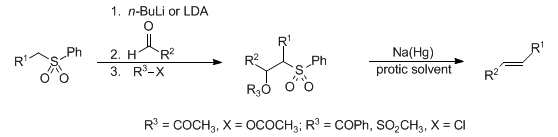
Reviews: P. Kocienski, Phosphorus Sulfur 24, 97-127 (1985); S. E. Kelly, Comp. Org. Syn. 1, 792-806. Synthetic applications: R. Bellingham et al., Synthesis 1996, 285; I. E. Markú et al., Tetrahedron Lett. 37, 2089 (1996); T. Satoh et al., ibid. 39, 6935 (1998); C. Charrier et al., ibid. 40, 5705 (1999). Modified conditions: P. R. Blakemore et al., Synthesis 7, 1209 (1999); P. J. Kocienski et al., Synlett 2000, 365.