Hofmann Reaction
A. W. Hofmann, Ber. 14, 2725 (1881).
Conversion of primary carboxylic amides to primary amines with one fewer carbon atom upon treatment with hypohalites or hydroxide via the intermediate isocyanate:
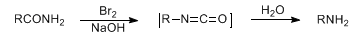
Early review: E. S. Wallis, J. F. Lane, Org. React. 3, 267-306 (1949). Alternative reagents/strategies: S. Kajigaeshi et al., Chem. Lett. 1989, 463; S. Jew et al., Arch. Pharmacal Res. 15, 333 (1992); D. S. Rane, M. M. Sharma, J. Chem. Technol. Biotechnol. 59, 271 (1994); H. Moustafa et al., Tetrahedron 53, 625 (1997); Y. Matsumura et al., J. Chem. Soc. Perkin Trans. 1 1999, 2057. Review: T. Shioiri, Comp. Org. Syn. 6, 800-806 (1991). Cf. Curtius Rearrangement; Lossen Rearrangement; Schmidt Reaction; Weerman Degradation.