Hantzsch Dihydropyridine Synthesis (Pyridine Synthesis)
A. Hantzsch, Ann. 215, 1, 72 (1882); Ber. 18, 1744 (1885); 19, 289 (1886).
Synthesis of dihydropyridines by condensation of two moles of a β-dicarbonyl compound with one mole of an aldehyde in the presence of ammonia. Dehydrogenation to the corresponding pyridine is accomplished with an oxidizing agent:
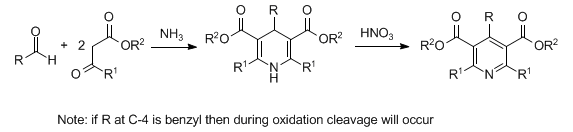
H. S. Mosher, Heterocycl. Compd. 1, 462 (1950); R. M. Kellog et al., J. Org. Chem. 45, 2854 (1980); Y. Watanabe et al., Synthesis 1983, 761. Mechanistic study: A. R. Katritzky et al., Tetrahedron 42, 5729 (1986); 43, 5171 (1987). Extension to the synthesis of unsymmetrical dihydropyridines: J. B. Sainani et al., Indian J. Chem. 34B, 17 (1995); S. Visentin et al., J. Med. Chem. 42, 1422 (1999). Cf. Chichibabin Pyridine Synthesis; Guareschi-Thorpe Condensation; Kröhnke Pyridine Synthesis.