Ferrier Rearrangement
R. J. Ferrier, J. Chem. Soc. Perkin Trans. 1 1979, 1455.
The stereochemically controlled conversion of hex-5-enopyranosides into cyclohexanones (inosose derivatives), catalyzed by mercury(II) salts, such that the 5-hydroxyl and the 3-substituent of the product are predominantly in a trans relationship:
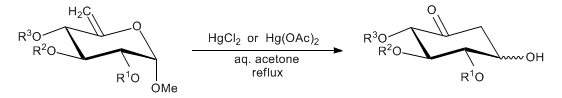
Stereochemical/mechanistic study: A. S. Machado et al., Carbohydr. Res. 233, C5 (1992); N. Yamauchi et al., Tetrahedron 50, 4125 (1994). Scope and limitations: N. Chida et al., Bull. Chem. Soc. Jpn. 64, 2118 (1991). Synthetic applications: D. H. R. Barton et al., Tetrahedron 46, 215 (1990); R. Chretien et al., Nat. Prod. Lett. 2, 69 (1993); A. B. Smith III et al., Org. Lett. 1, 909 (1999); eidem, ibid. 913. Modification of catalysis: J. C. López et al., J. Org. Chem. 60, 3851 (1995); T. Linker et al., Tetrahedron Lett. 39, 9637 (1998); B. S. Babu et al., Synth. Commun. 29, 4299 (1999); S. Hotha, A. Tripathi, Tetrahedron Lett. 46, 4555 (2005).