Favorskii Rearrangement; Wallach Degradation
A. E. Favorskii, J. Prakt. Chem. 88(2), 658 (1913); O. Wallach, Ann. 414, 296 (1918).
Base-catalyzed rearrangement of α-haloketones to acids or esters. The rearrangement of α,α′-dibromocyclohexanones to 1-hydroxycyclopentanecarboxylic acids, followed by oxidation to the ketones is known as the Wallach degradation:
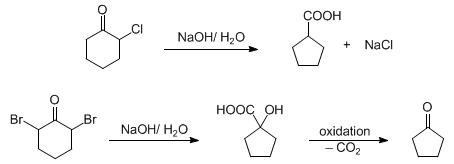
Detailed experimental procedure: D. W. Goheen, W. R. Vaughan, Org. Synth. coll. vol. 4, 594 (1963). Application to the synthesis of carboxylic acids: T. Satoh et al., Bull. Chem. Soc. Jpn. 66, 2339 (1993). Applications to asymmetric synthesis: idem et al., Tetrahedron Lett. 34, 4823 (1993); E. Lee, C. H. Yoon, Chem. Commun. 1994, 479. Reviews: A. S. Kende, Org. React. 11, 261-316 (1960); P. J. Chenier, J. Chem. Educ. 55, 286 (1978); A. Baretta, B. Waegill, “A Survey of Favorskii Rearrangement Mechanisms” in Reactive Intermediates, R. A. Abramovitch, Ed. (Plenum Press, New York, 1982) pp 527-585; J. Mann, Comp. Org. Syn. 3, 839-859 (1991).