Ene Reaction (Alder-Ene Reaction); Conia-Ene Reaction
K. Alder et al., Ber. 76, 27 (1943).
The addition of an alkene having an allylic hydrogen (ene) to a compound containing a multiple bond (enophile) to form a new bond between two unsaturated termini, with an allylic shift of the ene double bond, and transfer of the allylic hydrogen to the enophile. The mechanism is related to that of the Diels-Alder reaction, q.v.:
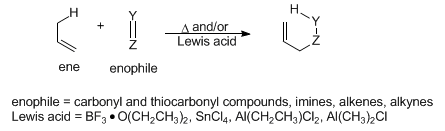
Lewis acid-promoted cyclization of 5-hexenals: J. A. Marshall, Chemtracts: Org. Chem. 5, 1-7 (1992). Review of alkenes as enophiles: B. B. Snider, Comp. Org. Syn. 5, 1-27 (1991). Review of carbonyl compounds as enophiles: idem, ibid. 2, 527-561; in conjunction with asymmetric synthesis: K. Mikami, M. Shimizu, Chem. Rev. 92, 1021-1050 (1992); K. Mikami et al., Synlett 1992, 255-265.
The intramolecular Ene reaction of unsaturated ketones, in which the carbonyl functionality serves as the ene component, via its tautomer, and the olefinic moiety serves as the enophile, is known as the Conia-Ene reaction: F. Rouessac et al., Tetrahedron Lett. 6, 3319 (1965).
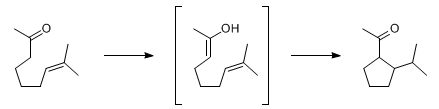
A. S. Kende, R. C. Newbold, Tetrahedron Lett. 30, 4329 (1989). J. J. Kennedy-Smith et al., J. Am. Chem. Soc. 126, 4526 (2004). Q. Gao et al., Org. Lett. 7, 2185 (2005). Review: J. M. Conia, P. Le Perchec, Synthesis 1975, 1-19.