D-Homo Rearrangement of Steroids
L. Ruzicka, H. Meldahl, Helv. Chim. Acta 21, 1760 (1938); 22, 421 (1939).
Originally discovered in 17β-hydroxy-20-ketosteroids, but thoroughly studied in the 17α-hydroxy-20-keto series, this reaction involves an acid- or base-catalyzed acyloin rearrangement which yields a 6-membered D-ring:
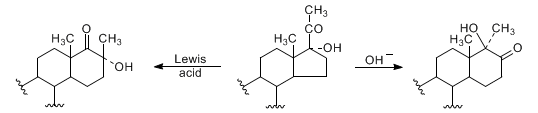
R. B. Turner, J. Am. Chem. Soc. 75, 3484 (1953); D. K. Fukushima et al., ibid. 77, 6585 (1955); N. L. Wendler et al., Tetrahedron 11, 163 (1960). Review: N. L. Wendler in Molecular Rearrangements Part 2, P. de Mayo, Ed. (Wiley-Interscience, New York, 1964) p 1114-1138. Extensive studies: D. Rabinovich et al., Chem. Commun. 1976, 461; N. G. Steinberg et al., J. Org. Chem. 49, 4731 (1984); L. Schor et al., J. Chem. Soc. Perkin Trans. 1 1990, 163; eidem, ibid. 1992, 453.