Dess-Martin Oxidation
D. B. Dess, J. C. Martin, J. Org. Chem. 48, 4155 (1983).
Mild oxidation of primary and secondary alcohols to aldehydes and ketones, respectively, employing the triacetoxyperiodinane (the “Dess-Martin Periodinane” reagent):
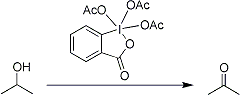
Scope and limitations of fluoroalkyl-substituted carbinols as substrates: R. J. Linderman, D. M. Graves, J. Org. Chem. 54, 661 (1989). Methods development: D. B. Dess, J. C. Martin, J. Am. Chem. Soc. 113, 7277 (1991). Application to the synthesis of 2′- and 3′-ketonucleosides: V. Samano, M. J. Robins, J. Org. Chem. 55, 5186 (1990); of substituted oxazoles: P. Wipf, C. P. Miller, ibid. 58, 3604 (1993). See monograph: Dess-Martin Periodinane.