Vorbrüggen Glycosylation
U. Niedballa, H. Vorbrüggen, Angew. Chem. Int. Ed. 9, 461 (1970).
The reaction of silylated heterocyclic bases with peracylated sugars in the presence of Lewis acids to yield natural β-nucleosides. If the sugar lacks a 2α-acyloxy substituent, an anomeric mixture forms:
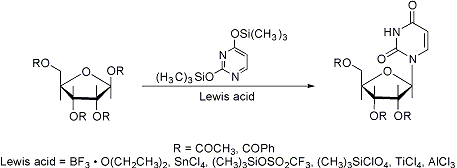
Scope and limitations: H. Vorbrüggen et al., Ber. 114, 1234 (1981). Mechanistic study: H. Vorbrüggen, G. Höfle, ibid. 1256. Synthetic applications: U. Niedballa, H. Vorbrüggen, J. Org. Chem. 39, 3654, 3660, 3664, 3668, 3672 (1974); R. O. Dempcy, E. B. Skibo, ibid. 56, 776 (1991); S. H. Kawai, G. Just, Nucleosides Nucleotides 10, 1485 (1991). Cf. Hilbert-Johnson Reaction.