Aldol Reaction (Condensation)
R. Kane, Ann. Phys. Chem., Ser. 2, 44, 475 (1838); idem, J. Prakt. Chem. 15, 129 (1838).
Traditionally, it is the acid- or base-catalyzed condensation of one carbonyl compound with the enolate/enol of another, which may or may not be the same, to generate a β-hydroxy carbonyl compound—an aldol. The method is compromised by self-condensation, polycondensation, generation of regioisomeric enols/enolates, and dehydration of the aldol followed by Michael addition, q.v. The development of methods for the preparation and use of preformed enolates or enol derivatives, that dictate specific carbon-carbon bond formation, have revolutionized the coupling of carbonyl compounds:
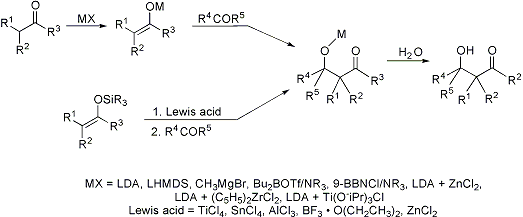
Historical perspective: C. H. Heathcock, Comp. Org. Syn. 2, 133-179 (1991). General review: T. Mukaiyama, Org. React. 28, 203-331 (1982). Application of lithium and magnesium enolates: C. H. Heathcock, Comp. Org. Syn. 2, 181-238 (1991); of boron enolates: B. M. Kim et al., ibid. 239-275; of transition metal enolates: I. Paterson, ibid. 301-319. Stereoselective reactions of ester and thioester enolates: M. Braun, H. Sacha, J. Prakt. Chem. 335, 653-668 (1993). Review of asymmetric methodology: A. S. Franklin, I. Paterson, Contemp. Org. Syn. 1, 317-338 (1994). Cf. Claisen-Schmidt Condensation; Henry Reaction; Ivanov Reaction; Knoevenagel Condensation; Reformatsky Reaction; Robinson Annulation.