Swern Oxidation (Moffatt-Swern Oxidation)
K. Omura, D. Swern, Tetrahedron 34, 1651 (1978).
Mild oxidation of primary and secondary alcohols, promoted by oxalyl chloride activation of dimethyl sulfoxide, evidently involving the dimethyl alkoxysulfonium salts. Upon the addition of base, the intermediates rearrange intramolecularly to generate aldehydes or ketones, respectively:
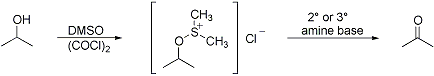
Reactivity/selectivity studies: M. Marx, T. T. Tidwell, J. Org. Chem. 49, 788 (1984). Reviews: A. J. Mancuso, D. Swern, Synthesis 1981, 165-185 passim; T. T. Tidwell, Org. React. 39, 297-572 passim (1990). Cf. Corey-Kim Oxidation; Pfitzner-Moffatt Oxidation.