Sonn-Müller Method
A. Sonn, E. Müller, Ber. 52, 1927 (1919).
Reaction sequence employed to convert aromatic anilides to aldehydes. Treatment of the anilide with phosphorus pentachloride generates the imidoyl chloride, which is reduced to the imine with a mixture of stannous chloride and hydrochloric acid. Subsequent hydrolysis yields the aldehyde:
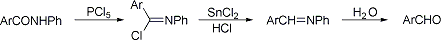
T. Reichstein, H. Zschokke, Helv. Chim. Acta 15, 1105 (1932); W. E. Bachmann, J. Am. Chem. Soc. 57, 1381 (1935); T. S. Work, J. Chem. Soc. 1942, 429; L. N. Ferguson, Chem. Rev. 38, 244 (1946); E. Mosettig, Org. React. 8, 240 (1954); L. F. Fieser, M. Fieser, Advanced Organic Chemistry (New York, 1961) p 832. Cf. Grundmann Aldehyde Synthesis; Stephen Aldehyde Synthesis.