Sandmeyer Diphenylurea Isatin Synthesis
T. Sandmeyer, Z. Farb. Textile Chem. 2, 129 (1903).
Formation of a cyanoformamidine by treatment of a symmetrical diphenylthiourea with potassium cyanide in alcohol containing lead carbonate, reduction with ammonium sulfide and ring-closure with concentrated sulfuric acid to isatin-2-anil; also formed smoothly by ring closure of the cyanoformamidine with aluminum chloride in benzene or carbon disulfide:
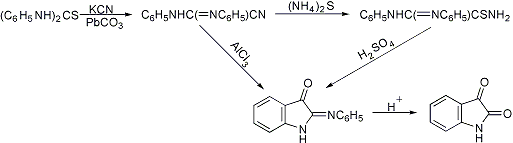
DE 115169, DE 116563 (both 1900 to J. R. Geigy & Co.); Friedländer 6, 574, 575 (1900-1902); A. Reissert, Ber. 37, 3708 (1904); G. Schultz et al., J. Prakt. Chem. [2] 74, 74, 76 (1906); C. Hollins, The Synthesis of Nitrogen Ring Compounds (London, 1924) p 102; C. S. Marvel, G. S. Hiers, Org. Syn. coll. vol. I, 327 (1943); P. L. Julian et al., Heterocyclic Compounds 3, 207 (1952); O. Bayer, W. Eckert, Houben-Weyl 7/4, 11 (1968). Cf. Sandmeyer Isonitrosoacetanilide Isatin Synthesis.