Mukaiyama Aldol Reaction
T. Mukaiyama et al., Chem Lett. 1973, 1011; idem et al., ibid. 1974, 323; eidem, J. Am. Chem. Soc. 96, 7503 (1974).
Formation of β-hydroxy ketones via reaction of silyl enol ethers or ketene silyl acetals with aldehydes in presence of a Lewis acid, such as titanium tetrachloride, tin tetrachloride or boron trifluoride etherate:
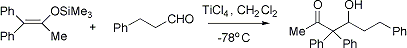
Enantioselectivity: E. M. Carreira et al., J. Am. Chem. Soc. 116, 8837 (1994). Diastereoselectivity: S. E. Denmark et al., Tetrahedron 54, 10389 (1998). Reviews: H. Gröger et al., Chem. Eur. J. 4, 1137-1141 (1998); E. M. Carreira in Comprehensive Asymmetric Catalysis I-III vol. 3, E. N. Jacobsen et al., Eds. (Springer-Verlag, Berlin, Germany, 1999) 997-1065; K. Iseki, ACS Symp. Ser. 746, 38-51 (2000). Cf. Aldol Reaction; Evans Aldol Reaction.