Mislow-Evans Rearrangement
P. Bickart et al., J. Am. Chem. Soc. 90, 4869 (1968); D. A. Evans et al., ibid. 93, 4956 (1971).
[2,3]-Sigmatropic rearrangement of allylic sulfoxides to allylic sulfenates which are captured by thiophiles to generate the allylic alcohols, thereby effecting the 1,3-transposition of sulfoxide and alcohol functions. The reverse process is accomplished by treating the alcohol with arylsulfenyl chloride, followed by thermal rearrangement of the sulfenate to generate the allylic sulfoxide:
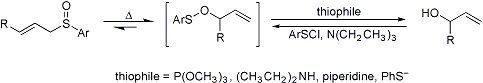
Early review: D. A. Evans, G. C. Andrews, Accts. Chem. Res. 7, 147 (1974). Acid-catalyzed modification: Y. Masaki et al., Chem. Pharm. Bull. 33, 2531 (1985). Synthetic applications: H. J. Reich, S. Wollowitz, J. Am. Chem. Soc. 104, 7051 (1982); G. H. Posner et al., J. Org. Chem. 52, 4836 (1987); A. Padwa et al., ibid. 56, 4252 (1991). Mechanistic studies: D. K. Jones-Hertzog, W. L. Jorgensen, ibid. 60, 6682 (1995); eidem, J. Am. Chem. Soc. 117, 9077 (1995). Cf. Meisenheimer Rearrangements; [2,3]-Wittig Rearrangement.