Miescher Degradation
C. Meystre et al., Helv. Chim. Acta 27, 1815 (1944).
Adaptation of the Barbier-Wieland degradation, q.v., to permit simultaneous elimination of three carbon atoms, as in degradation of the bile acid side chain to the methyl ketone stage. Conversion of the methyl ester of the bile acid to the tertiary alcohol, followed by dehydration, bromination, dehydrohalogenation and oxidation of the diene yields the chain-shortened ketone:
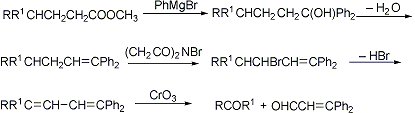
C. W. Shoppee, Ann. Repts. (Chem. Soc. London) 44, 184 (1947); F. S. Spring, J. Chem. Soc. 1950, 3355; A. Wettstein, G. Anner, Experientia 1954, 407; C. J. W. Brooks, Rodd's Chemistry of Carbon Compounds IID, 26 (1970); P. G. Marshall, ibid. 233, 253, 323.