Meisenheimer Rearrangements
J. Meisenheimer, Ber. 52, 1667 (1919).
Formation of O, N, N-trisubstituted hydroxylamines from tertiary amine oxides via [1,2]-R group migration, or [2,3]-sigmatropic rearrangement when R′ = allyl:
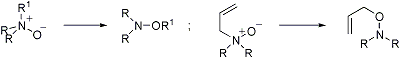
[1,2]-Rearrangements: N. Castagnoli, Jr. et al., Tetrahedron 26, 4319 (1970); J. B. Bremner et al., Aust. J. Chem. 41, 293 (1988); R. Yoneda et al., Tetrahedron Letters 35, 3749 (1994); eidem, Tetrahedron 52, 14563 (1996). Cf. Stevens Rearrangement; [1,2]-Wittig Rearrangement.
[2,3]-Rearrangements: V. Rautenstrauch, Helv. Chim. Acta 56, 2492 (1973); Y. Yamamato et al., J. Org. Chem. 41, 303 (1976); or [1,2]: T. Kurihara et al., Chem. Pharm. Bull. 42, 475 (1994). Asymmetric syntheses: D. Enders, H. Kempen, Synlett. 1994, 969; S. G. Davies, G. D. Smyth, Tetrahedron Asymmetry 7, 1001 (1996); J. E. H. Buston et al., ibid. 9, 1995 (1998). Cf. Mislow-Evans Rearrangement; Sommelet-Hauser Rearrangement; [2,3]-Wittig Rearrangement.