Malonic Ester Syntheses
Syntheses based on the strongly activated methylene group of malonic esters which on reaction with sodium ethoxide form a resonance-stabilized ion that can be alkylated or acylated. After hydrolysis, the free alkylmalonic acids readily decarboxylate to mono- or disubstituted monocarboxylic acids:
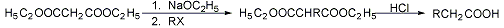
H. O. House, Modern Synthetic Reactions (W. A. Benjamin, Menlo Park, California, 2nd ed., 1972) pp 510-518, 756-761. Use of crown ethers as catalysts: D. H. Hunter, et al., Synthesis 1977, 37. Modified conditions: M. A. Casadei et al., J. Org. Chem. 46, 3127 (1981); B. K. Wilk, Synth. Commun. 26, 3859 (1996). Stereoselectivity: T. Sato, J. Otera, J. Org. Chem. 60, 2627 (1995); B. Klotz-Berendes et al., Tetrahedron Asymmetry 8, 1821 (1997). Cf. Perkin Alicyclic Synthesis.