Leuckart (Leukart) Reaction; Leuckart-Wallach Reaction; Eschweiler-Clarke Reaction
R. Leuckart, Ber. 18, 2341 (1885).
Reductive alkylation of ammonium (or amine) salts of formic acid or formamides by aldehydes or ketones:
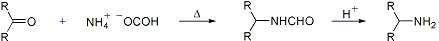
When the reaction is performed in the presence of excess formic acid it is referred to as the Leuckart-Wallach reaction: O. Wallach, Ann. 272, 99 (1892). Application to steroids: W. E. Solomons, N. J. Doorenbos, J. Pharm. Sci. 63, 19 (1974); A. M. Bellini et al., Steroids 56, 395 (1991).
The reductive methylation of primary or secondary amines employing formaldehyde and formic acid is known as the Eschweiler-Clarke reaction: W. Eschweiler, Ber. 38, 880 (1905); H. T. Clarke, et al., J. Am. Chem. Soc. 55, 4571 (1933). Synthetic applications: E. Farkas, C. J. Sunman, J. Org. Chem. 50, 1110 (1985); J. Casanova, P. Devi, Synth. Commun. 23, 245 (1993).
Early reviews: M. L. Moore, Org. React. 5, 301-330 (1949); F. Möller, R. Schröter, Houben-Weyl 11/1, 648-664 (1957). Application to deoxybenzoins: M. J. Villa et al., Heterocycles 24, 1943 (1986). Mechanistic study: P. I. Awachie, V. C. Agwada, Tetrahedron 46, 1899 (1990); A. G. Martinez et al., Tetrahedron Asymmetry 10, 1499 (1999). Optimized procedure: R. Carlson et al., Acta Chem. Scand. 47, 1046 (1993). Modified conditions: A. Loupy et al., Tetrahedron Letters 37, 8177 (1996); I. Helland, T. Lejon, Heterocycles 51, 611 (1999).