Barbier-Wieland Degradation
H. Wieland, Ber. 45, 484 (1912); P. Barbier, R. Locquin, Compt. Rend. 156, 1443 (1913).
Stepwise carboxylic acid degradation of aliphatic acids (particularly in sterol side chains) to the next lower homolog. The ester is converted to a tertiary alcohol that is dehydrated with acetic anhydride, and the olefin oxidized with chromic acid to a lower homologous carboxylic acid:
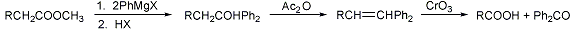
H. Wieland et al., Z. Physiol. Chem. 161, 80 (1926); C. W. Shoppee, Ann. Repts. (Chem. Soc., London) 44, 184 (1947); W. Baker et al., J. Chem. Soc. 1958, 1007; J. R. Dias, R. Ramachandra, Tetrahedron Letters 1976, 3685. Synthetic applications: S. C. Wilcox, J. J. Guadino, J. Am. Chem. Soc. 108, 3102 (1986); C. D. Schteingart, A. E. Hofmann, J. Lipid Res. 29, 1387 (1988). Cf. Krafft Degradation; Miescher Degradation.