Glaser Coupling; Eglinton Reaction; Cadiot-Chodkiewicz Coupling
C. Glaser, Ber. 2, 422 (1869).
Homocoupling of terminal alkynes catalyzed by cuprous salts in the presence of an oxidant and ammonium chloride:
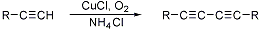
Synthetic applications: F. M. Menger et al., J. Am. Chem. Soc. 115, 6600 (1993); L. Guo et al., Chem. Commun. 1994, 243.
This coupling may also be effected by cupric salts in pyridine and is often referred to as the Eglinton reaction. It is particularly applicable to cyclizations: G. Eglinton, A. R. Galbraith, Chem. & Ind. (London) 1956, 737; N. Hébert et al., J. Org. Chem. 57, 1777 (1992).
Heterocoupling may be accomplished via the Cadiot-Chodkiewicz coupling of terminal alkynes with haloalkynes, catalyzed by cuprous salts in the presence of aliphatic amines:
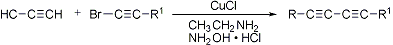
W. Chodkiewicz et al., Compt. Rend. 245, 322 (1957); B. N. Ghose, Syn. React. Inorg. Met.-Org. Chem. 24, 29 (1994); with supercritical CO2 as solvent: J. Li, H. Jiang, Chem. Commun. 1999, 2369.
Inclusive reviews: P. Cadiot, W. Chodkiewicz, “Couplings of Acetylenes” in Chemistry of Acetylenes, H. G. Viehe, Ed. (Marcel Dekker, New York, 1969) pp 597-647; K. Sonogashira, Comp. Org. Syn. 3, 551-561 (1991). Cf. Castro-Stephens Coupling; Ullmann Reaction.