Freund Reaction; Gustavson Reaction; Hass Cyclopropane Process
A. Freund, Monatsh. 3, 625 (1882); G. Gustavson, J. Prakt. Chem. [2] 36, 300 (1887); H. B. Hass et al., Ind. Eng. Chem. 28, 1178 (1936).
Formation of alicyclic hydrocarbons by the action of sodium (Freund reaction) or zinc (Gustavson reaction) on open chain dihalo compounds; 1,3-dichloropropane derived from the chlorination of propane obtained from natural gas is cyclized in the Hass cyclopropane process by treating with zinc dust in aqueous alcohol in the presence of catalytic sodium iodide:
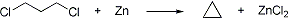
H. Gilman, Organic Chemistry I (New York, 1943) p 74; J. D. Bartleson et al., J. Am. Chem. Soc. 68, 2513 (1946); R. N. Shortsidge et al., ibid. 70, 946 (1948); B. T. Brooks, The Chemistry of the Nonbenzenoid Hydrocarbons (New York, 1950) p 88; H. F. Ebel, A. Lüttringhaus, Houben-Weyl 13/1, 492 (1970).