Feist-Bénary Synthesis
F. Feist, Ber. 35, 1537, 1545 (1902); E. Bénary, Ber. 44, 489, 493 (1911).
Formation of furans from α-halogenated ketones or ethers and 1,3-dicarbonyl compounds in the presence of pyridine. When ammonia is used as the condensing agent, pyrrole derivatives are always formed as secondary products:
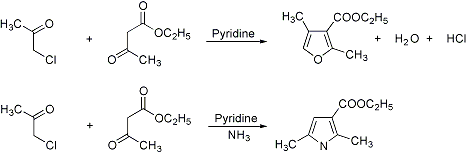
T. Reichstein, H. Zschokke, Helv. Chim. Acta 14, 1270 (1931); 15, 268, 1105, 1112 (1932); R. C. Elderfield, T. N. Dodd, Heterocyclic Compounds 1, 132 (1950); J. Kagan, K. C. Mattes, J. Org. Chem. 45, 1524 (1980). Alternative substrate: R. C. Cambie et al., Synth. Commun. 20, 1923 (1990). Cf. Hantzsch Pyrrole Synthesis.