Doebner-Miller Reaction; Beyer Method for Quinolines
O. Doebner, W. v. Miller, Ber. 16, 2464 (1883).
Acid-catalyzed synthesis of quinolines from primary aromatic amines and α,β-unsaturated carbonyl compounds. When the latter are prepared in situ from two molecules of aldehyde or an aldehyde and methyl ketone, the reaction is known as the Beyer method for quinolines:
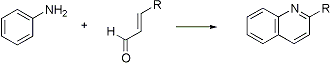
F. W. Bergström, Chem. Rev. 35, 153 (1944); Y. Ogata et al., J. Chem. Soc. B 1969, 805; G. A. Dauphinee, T. P. Forrest, J. Chem. Soc. D 1969, 327; Can. J. Chem. 56, 632 (1978); C. M. Leir, J. Org. Chem. 42, 911 (1977). Applications: G. K. Lund et al., J. Chem. Eng. Data 26, 227 (1981); W. Buchowiecki et al., J. Prakt. Chem. 327, 1015 (1985); T. Blitzke et al., ibid. 335, 683 (1993). Cf. Gould-Jacobs Reaction; Knorr Quinoline Synthesis.