Barton Olefin Synthesis (Barton-Kellogg Reaction)
D. H. R. Barton et al., Chem. Commun. 1970, 1226; R. M. Kellogg, S. Wassenaar, Tetrahedron Letters 1970, 1987; R. M. Kellogg et al., ibid. 4689.
Olefin synthesis by two-fold extrusion of nitrogen and sulfur from a Δ3-1,3,4-thiadiazoline intermediate. Particularly applicable to the synthesis of moderately hindered tetra-substituted ethylenes:
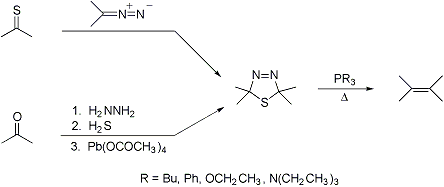
Scope and limitations: D. H. R. Barton et al., J. Chem. Soc. Perkin Trans. I 1974, 1794. Synthetic applications: A. P. Schaap, G. R. Faler, J. Org. Chem. 38, 3061 (1973); L. K. Bee et al., ibid. 40, 2212 (1975); M. D. Bachi et al., Tetrahedron Letters 1978, 4167; J. E. McMurry et al., J. Am. Chem. Soc. 106, 5018 (1984); F. J. Hoogesteger et al., J. Org. Chem. 60, 4375 (1995). Cf. McMurry Reaction.