An essential component of Injections preserved in multiple-dose containers is the agent or agents present to reduce the hazard of having introduced, in the course of removing some of the contents, accidental microbial contamination of the contents remaining. It is a Pharmacopeial requirement that the presence and amount added of such agent(s) be declared on the label of the container. The methods provided herein for the most commonly used agents are to be used to demonstrate that the declared agent is present but does not exceed the labeled amount by more than 20% of the labeled amount.
The concentration of an antimicrobial preservative added to a multiple-dose or single-dose parenteral, otic, nasal, and ophthalmic preparation may diminish during the shelf life of the product. Because it is recognized that the antimicrobial preservative concentration in a given preparation may decrease during the product's shelf life, the manufacturer shall determine the lowest level at which the preservative is effective, and the product should be so formulated as to assure that this level is exceeded throughout the product's shelf life. At the time of its manufacture, the product should contain the declared amount of antimicrobial preservative (within ±20% to allow for manufacturing and analytical variations). The quantitative label statement of the preservative content is not intended to mean that the labeled quantity is retained during the shelf life of the product; rather, it is a statement of the amount added, within process limits, and which is not exceeded by more than 20%. An example of such a label statement is “____(unit) added as preservative.” [note—“____(unit)” would be a number followed by the unit of measurement, e.g., 0.015 mg per mL or 0.1%.]
The most commonly used agents include the two mercurials, phenylmercuric nitrate and thimerosal and the four homologous esters of p-hydroxybenzoic acid, phenol, benzyl alcohol, and chlorobutanol. The methods for the first two named are polarographic, while quantitative gas chromatography is employed in the determination of the other agents.
GENERAL GAS CHROMATOGRAPHIC METHOD
The general procedures set forth in the following paragraphs are applicable to the quantitative determination of benzyl alcohol, chlorobutanol, phenol, and the methyl, ethyl, propyl, and butyl esters of p-hydroxybenzoic acid, the latter being treated as a group, the individual members of which, if present, are capable of separate determination. Prepare the Internal Standard Solution and the Standard Preparation for each agent as directed individually below. Unless otherwise directed below, prepare the Test Preparation from accurately measured portions of the Internal Standard Solution and the sample under test, of such size that the concentration of the agent and the composition of the solvent correspond closely to the concentration and composition of the Standard Preparation. Suggested operating parameters of the gas chromatograph apparatus are given in the accompanying table, the carrier gas being helium or nitrogen, and the detector being the flame-ionization type.
Suggested Operating Parameters of Gas Chromatograph Apparatus
| Agent | Column Size | Column Packing Phases and Support | Flow Rate, mL per minute | Column Temperature | |
| Length | ID | ||||
| Benzyl Alcohol | 1.8 m | 3 mm | 5% G16/S1A | 50 | 140 |
| Chlorobutanol | 1.8 m | 2 mm | 5% G16/S1A | 20 | 110 |
| Phenol | 1.2 m | 3 mm | 5% G16/S1A | 50 | 145 |
| Parabens | 1.8 m | 2 mm | 5% G2/S1A | 20 | 150 |
Benzyl Alcohol
Internal Standard Solution—
Dissolve about 380 mg of phenol in 10 mL of methanol contained in a 200-mL volumetric flask. Add water to volume, and mix.
Standard Preparation—
Dissolve about 180 mg of USP Benzyl Alcohol RS, accurately weighed, in 20.0 mL of methanol contained in a 100-mL volumetric flask. Add Internal Standard Solution to volume, and mix.
Procedure—
Separately inject equal volumes (about 5 µL) of the Standard Preparation and the Test Preparation into the chromatograph, record the chromatograms with the apparatus adjusted to the parameters set forth in the accompanying table, and measure the areas under the peaks for benzyl alcohol and phenol. Calculate the content, in mg per mL, of benzyl alcohol (C7H8O) in the specimen taken by the formula:
100(C/V)(p1 / p2)(P2 / P1)
in which C is the concentration, in mg per mL, of benzyl alcohol in the Standard Preparation; V is the volume, in mL, of the specimen under test used in preparing each 100 mL of the Test Preparation; p1 and p2 are the peak areas for benzyl alcohol and phenol, respectively, obtained from the Test Preparation; and P1 and P2 are the peak areas of benzyl alcohol and phenol, respectively, obtained from the Standard Preparation.
Chlorobutanol
Internal Standard Solution—
Transfer about 140 mg of benzaldehyde to a 100-mL volumetric flask, add 10 mL of methanol, and swirl to dissolve. Dilute with water to volume, and mix.
Standard Preparation—
Transfer about 125 mg of USP Chlorobutanol RS, accurately weighed, to a 25-mL volumetric flask. Add 2 mL of methanol, swirl to dissolve, dilute with water to volume, and mix. Transfer 5.0 mL of this solution and 5.0 mL of Internal Standard Solution to a 25-mL flask, and mix to obtain a solution having a known concentration of about 2.5 mg of chlorobutanol per mL.
Test Preparation—
Quantitatively dilute, if necessary, an accurately measured volume of the specimen under test with methanol to obtain a solution containing not more than about 5.0 mg of chlorobutanol per mL. Combine 3.0 mL of this solution with 3.0 mL of Internal Standard Solution, and mix.
Chromatographic System (see Chromatography  621
621 )—
[note—See accompanying table for column dimensions, column packing phase and support, flow rate, and column temperature.] The injection port temperature is maintained at 180
)—
[note—See accompanying table for column dimensions, column packing phase and support, flow rate, and column temperature.] The injection port temperature is maintained at 180 , and the detector temperature is maintained at 220
, and the detector temperature is maintained at 220 . Chromatograph the Standard Preparation, and record the peak responses as directed for Procedure: the relative retention times are about 0.8 for benzaldehyde and 1.0 for chlorobutanol; the resolution, R, between benzaldehyde and the chlorobutanol is not less than 2.0; and the relative standard deviation for replicate injections is not more than 2.0%.
. Chromatograph the Standard Preparation, and record the peak responses as directed for Procedure: the relative retention times are about 0.8 for benzaldehyde and 1.0 for chlorobutanol; the resolution, R, between benzaldehyde and the chlorobutanol is not less than 2.0; and the relative standard deviation for replicate injections is not more than 2.0%.
Procedure—
Separately inject equal volumes (about 1 µL) of the Standard Preparation and the Test Preparation into the chromatograph, record the chromatograms, and measure the areas for the major peaks. Calculate the quantity, in mg, of chlorobutanol (C4H7Cl3O) in each mL of the specimen under test by the formula:
C(L/D)(RU / RS)
in which C is the concentration, in mg per mL, of chlorobutanol, calculated on the anhydrous basis, in the Standard Preparation; L is the labeled quantity, in mg, of chlorobutanol in each mL of the specimen under test; D is the concentration, in mg per mL, of chlorobutanol in the Test Preparation, based on the volume of specimen under test taken and the extent of dilution; and RU and RS are the ratios of the chlorobutanol peak to the benzaldehyde peak obtained from the Test Preparation and the Standard Preparation, respectively.
Phenol
Internal Standard Solution—
Pipet 1 mL of USP Benzyl Alcohol RS into a 500-mL volumetric flask, add methanol to volume, and mix.
Standard Preparation—
Dissolve about 75 mg of USP Phenol RS, accurately weighed, in 7.5 mL of methanol contained in a 100-mL volumetric flask. Add 20.0 mL of Internal Standard Solution, then add water to volume, and mix.
Procedure—
Separately inject equal volumes (about 3 µL) of the Standard Preparation and the Test Preparation into the chromatograph, record the chromatograms with the apparatus adjusted to the parameters set forth in the accompanying table, and measure the areas under the peaks for phenol and benzyl alcohol. Calculate the content, in mg per mL, of phenol (C6H6O) in each mL of the specimen taken by the formula:
100(C/V)(p1 / p2)(P2 / P1)
in which C is the concentration, in mg per mL, of phenol in the Standard Preparation; V is the volume, in mL, of the specimen under test used in preparing each 100 mL of the Test Preparation; p1 and p2 are the peak areas for phenol and benzyl alcohol, respectively, obtained from the Test Preparation; and P1 and P2 are the peak areas of phenol and benzyl alcohol, respectively, obtained from the Standard Preparation.
Methylparaben and Propylparaben
Internal Standard Solution—
Place about 200 mg of benzophenone in a 250-mL volumetric flask, dilute with ether to volume, and mix.
Standard Preparation—
Place 100 mg of USP Methylparaben RS and 10 mg of USP Propylparaben RS, each accurately weighed, in a 200-mL volumetric flask, dilute with Internal Standard Solution to volume, and mix. Place 10 mL of this solution in a 25-mL conical flask, and proceed as directed for Test Preparation, beginning with “Add 3 mL of pyridine.”
Test Preparation—
Pipet 10 mL of the specimen under test and 10 mL of the Internal Standard Solution into a small separator. Shake vigorously, allow the layers to separate, draw off the aqueous layer into a second separator, and transfer the ether layer into a small flask through a funnel containing anhydrous sodium sulfate. Extract the aqueous layer with two 10-mL portions of ether, also filtering the extracts through the anhydrous sodium sulfate. Evaporate the combined extracts under a current of dry air until the volume is reduced to about 10 mL, then transfer the residue to a 25-mL conical flask. Add 3 mL of pyridine, complete the evaporation of the ether, and boil on a hot plate until the volume is reduced to about 1 mL. Cool, and add 1 mL of a suitable silylation agent, such as bis(trimethylsilyl)trifluoroacetamide, bis(trimethylsilyl)acetamide, or a mixture of hexamethyldisilazane and trimethylchlorosilane [2:1 or 3:1 (v/v)]. Mix, and allow to stand for not less than 15 minutes.
Procedure—
Separately inject equal volumes (2 µL) of the silanized solution from the Standard Preparation and the Test Preparation into the chromatograph, record the chromatograms with the apparatus adjusted to the parameters set forth in the accompanying table, and measure the areas under the peaks for methylparaben, propylparaben, and benzophenone. Calculate the content, in µg per mL, of methylparaben (C8H8O3) in the sample under test by the formula:
10(CM / V)(p1 / p3)(P3 / P1)
in which CM is the concentration, in µg per mL, of methylparaben in the Standard Preparation; V is the volume, in mL, of the specimen taken; p1 and p3 are the peak areas for methylparaben and benzophenone, respectively, obtained from the Test Preparation; and P1 and P3 are the peak areas of methylparaben and benzophenone, respectively, obtained from the Standard Preparation. Similarly, calculate the content, in µg per mL, of propylparaben (C10H12O3) in the specimen under test by the formula:
10(CP / V)(p2 / p3)(P3 / P2)
in which CP is the concentration, in µg per mL, of propylparaben in the Standard Preparation; V is the volume, in mL, of the specimen taken; p2 and p3 are the peak areas for propylparaben and benzophenone, respectively, obtained from the Test Preparation; and P2 and P3 are the peak areas of propylparaben and benzophenone, respectively, obtained from the Standard Preparation.
Ethylparaben and butylparaben may be determined in a similar manner.
POLAROGRAPHIC METHOD
Phenylmercuric Nitrate
Standard Preparation—
Dissolve about 100 mg of phenylmercuric nitrate, accurately weighed, in sodium hydroxide solution (1 in 250) contained in a 1000-mL volumetric flask, warming if necessary to effect solution, add the sodium hydroxide solution to volume, and mix. Pipet 10 mL of this solution into a 25-mL volumetric flask, and proceed as directed under Test Preparation, beginning with “add 2 mL of potassium nitrate solution (1 in 100).”
Test Preparation—
Pipet 10 mL of the specimen under test into a 25-mL volumetric flask, add 2 mL of potassium nitrate solution (1 in 100) and 10 mL of pH 9.2 alkaline borate buffer (see under Buffer Solutions in the section Reagents, Indicators, and Solutions), and adjust to a pH of 9.2, if necessary, by the addition of 2 N nitric acid. Add 1.5 mL of freshly prepared gelatin solution (1 in 1000), then add the pH 9.2 alkaline borate buffer to volume, and mix.
Procedure—
Pipet a portion of the Test Preparation into the polarographic cell, and deaerate by bubbling nitrogen through the solution for 15 minutes. Insert the dropping mercury electrode of a suitable polarograph (see Polarography  801
801 ), and record the polarogram from
), and record the polarogram from 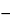 0.6 to
0.6 to 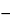 1.5 volts versus the saturated calomel electrode. Determine the diffusion current of the Test Preparation, (id)U, as the difference between the residual current and the limiting current. Similarly and concomitantly determine the diffusion current, (id)S, of the Standard Preparation. Calculate the quantity, in µg, of phenylmercuric nitrate (C6H5HgNO3) in each mL of the specimen taken by the formula:
1.5 volts versus the saturated calomel electrode. Determine the diffusion current of the Test Preparation, (id)U, as the difference between the residual current and the limiting current. Similarly and concomitantly determine the diffusion current, (id)S, of the Standard Preparation. Calculate the quantity, in µg, of phenylmercuric nitrate (C6H5HgNO3) in each mL of the specimen taken by the formula:
2.5C[(id)U / (id)S]
in which C is the concentration, in µg per mL, of phenylmercuric nitrate in the Standard Preparation.
Thimerosal
Standard Preparation—
On the day of use, place about 25 mg of USP Thimerosal RS, accurately weighed, in a 250-mL volumetric flask, add water to volume, and mix. Protect from light. Pipet 15 mL of this solution into a 25-mL volumetric flask, add 1.5 mL of gelatin solution (1 in 1000), then add potassium nitrate solution (1 in 100) to volume, and mix.
Test Preparation—
Pipet 15 mL of the test specimen into a 25-mL volumetric flask, add 1.5 mL of gelatin solution (1 in 1000), add potassium nitrate solution (1 in 100) to volume, and mix.
Procedure—
Transfer a portion of the Test Preparation to a polarographic cell, and deaerate by bubbling nitrogen through the solution for 15 minutes. Insert the dropping mercury electrode of a suitable polarograph (see Polarography 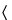 801
801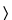 ), and record the polarogram from
), and record the polarogram from 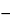 0.2 to
0.2 to 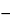 1.4 volts versus the saturated calomel electrode. Determine the diffusion current, (id)U, as the difference between the residual current and the limiting current. Similarly and concomitantly determine the diffusion current, (id)S, of the Standard Preparation. Calculate the quantity, in µg, of thimerosal (C6H9HgNaO2S) in each mL of the test specimen taken by the formula:
1.4 volts versus the saturated calomel electrode. Determine the diffusion current, (id)U, as the difference between the residual current and the limiting current. Similarly and concomitantly determine the diffusion current, (id)S, of the Standard Preparation. Calculate the quantity, in µg, of thimerosal (C6H9HgNaO2S) in each mL of the test specimen taken by the formula:
1.667C[(id)U / (id)S]
in which C is the concentration, in µg per mL, of thimerosal in the Standard Preparation; and the other terms are as defined therein.
Auxiliary Information— Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
| General Chapter | Behnam Davani, Ph.D., M.B.A.
Senior Scientist 1-301-816-8394 |
(MDAA05) Monograph Development-Antivirals and Antimicrobials |
USP32–NF27 Page 141
Pharmacopeial Forum: Volume No. 30(5) Page 1678