PROCESSING
Fetal bovine serum (FBS) is the light-brown liquid fraction of clotted fetal bovine blood. It is depleted of cells, fibrin, and clotting factors. Although the complete composition of FBS is undefined, FBS contains high levels of growth factors and low levels of immunoglobulins. In addition, it contains other key ingredients that are essential in supporting proliferation of cells in culture. This product is used both in life science basic research and industrial manufacturing. FBS is a by-product of the meat industry and is collected from bovine fetuses removed from cattle found to be pregnant at slaughter. FBS is harvested from abattoirs that are inspected by the competent authority in the country of origin. Trained personnel following written and approved procedures should perform collection and processing. Blood is collected in a closed system in a dedicated area within the facility, and processed quickly to prevent hemolysis. The blood is allowed to clot and then typically is centrifuged in a refrigerated centrifuge to separate the serum from the other components. Serum typically is removed from the clot, transferred to labeled containers, and frozen. All manufacturers employ sterile filtration before final packaging. Additionally, gamma irradiation provides the highest assurance of the absence of viral activity. Gamma irradiation doses of 25–40 kGy provide significant log reduction of viral and other adventitious agents while preserving cellular growth performance.
The screening of FBS for viral contamination is accomplished by using all applicable testing described in the Code of Federal Regulations 9 CFR 113.53 (known as full 9 CFR testing). Mycoplasma assays are performed as described in Mycoplasma Tests  63
63 .
.
FETAL BOVINE SERUM QUALITY ATTRIBUTES
Packaging and Storage:
Store in sealed containers at a temperature of  10
10 or below.
or below.
Labeling:
Label it to indicate that contents are Fetal Bovine Serum, and indicate lot number, expiration date, and storage conditions. Also, indicate country of origin on product labeling.
pH  791
791 :
7.00–8.00, in undiluted serum samples
:
7.00–8.00, in undiluted serum samples
Osmolality  785
785 :
280–360 mOsmol/kg
:
280–360 mOsmol/kg
Bacterial Endotoxins  85
85 :
It contains not more than 10 USP Endotoxin Units/mL of serum.
:
It contains not more than 10 USP Endotoxin Units/mL of serum.
Total Protein Content  1057
1057 :
30–45 mg/mL
:
30–45 mg/mL
Sterility Tests  71
71 :
Meets the requirements
:
Meets the requirements
Identification—Radial Immunodiffusion
Reagents
— FBS test samples
— Horse serum, negative control samples
— Bovine IgG calibrator (500 mg/L)
— Sheep albumin diluent (1% Sheep albumin, 0.18% EDTA, 1.75% NaCl, and 1.21% Tris/HCl pH 7.4).
Materials/Apparatus:
Ring measuring device is calibrated in 0.1-mm increments. Radial immunodiffusion (RID) plates are commercially available and contain anti-bovine IgG antiserum in a 1.5% agarose gel, 0.1 M phosphate buffer, pH 7.0, 0.1% sodium azide as bacteriostatic agent, and 1 µg/mL amphotericin B as an antifungal agent. Store at 2 –8
–8 . Use RID plates that can measure bovine IgG in the range of 50–500 mg/L.
. Use RID plates that can measure bovine IgG in the range of 50–500 mg/L.
Standard curve:
Use the bovine IgG calibrators for system suitability and for generation of a calibration curve. Prepare two dilutions from a 500 mg/L bovine IgG stock solution. Dilute 120 µL of the 500 mg/L stock with 80 µL of diluent (medium dilution) and 25 µL of the 500 mg/L stock with 225 µL diluent (low dilution). Label each dilution respectively as 300 mg/L and 50 mg/L calibrators. Use the 500 mg/L, 300 mg/L, and 50 mg/L solutions to generate the standard curve. [Note—Prepare and analyse the calibrator bovine IgG solutions in duplicate. ] Load 5 µL of each sample into the 2.5-mm wells of the plate. At 72 h of incubation, measure ring diameters to the nearest 0.1 mm using an appropriate ring measuring device. Record the results and proceed to the generation of a standard curve.
The ring diameter should develop to completion at room temperature for 72 h. Using the result from each data point of the standard curve, generate a single linearity plot where y is the squared diameter (mm2) of precipitin ring around the well and x is the Bovine IgG concentration (mg/L). Calculate the linear least-squares-fit regression line of the form y = m(x) + b with the help of suitable software and determine the values for slope (m), y-intercept (b), and coefficient of determination (R2). The standard curve for the method is linear if R2 is 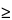 0.98.
0.98.
Analysis:
Frozen undiluted samples of FBS are thawed and tested within 24 h if stored at 4 . Testing of FBS test and USP Fetal Bovine Serum RS samples is performed in triplicate. Prepare RID plates containing anti-bovine IgG to be tested for the various types of sera. Allow plates and reagents to equilibrate to room temperature before use by leaving the plates open for 10–15 min at room temperature to allow any condensation in the wells or on the gel surface to evaporate. Samples should not be applied to wells where moisture is visible. Prepare serial dilutions, if necessary, of FBS test and USP Fetal Bovine Serum RS samples in diluent. Dilute the negative control horse serum in diluent. Load 5 µL of each sample into the 2.5-mm wells of the plate, and incubate at room temperature for 72 h. [Note—The test samples and the negative control are loaded on the same plate. ]
. Testing of FBS test and USP Fetal Bovine Serum RS samples is performed in triplicate. Prepare RID plates containing anti-bovine IgG to be tested for the various types of sera. Allow plates and reagents to equilibrate to room temperature before use by leaving the plates open for 10–15 min at room temperature to allow any condensation in the wells or on the gel surface to evaporate. Samples should not be applied to wells where moisture is visible. Prepare serial dilutions, if necessary, of FBS test and USP Fetal Bovine Serum RS samples in diluent. Dilute the negative control horse serum in diluent. Load 5 µL of each sample into the 2.5-mm wells of the plate, and incubate at room temperature for 72 h. [Note—The test samples and the negative control are loaded on the same plate. ]
Calculation:
After 72 h, measure the diameters of the rings using the ring measuring device, and record the results. Using the regression equation developed under standard curve deviation, calculate the concentration of bovine IgG in FBS samples. Concentration is expressed as mg/L.
Acceptance criteria:
Horse serum is negative (should not give a precipitation ring). FBS test and USP Fetal Bovine Serum RS samples are positive and contain NMT 500 mg/L of IgG.
Hemoglobin content:
Sample preparation:
FBS samples are thawed, are stored at 4 , and are tested within the same day.
, and are tested within the same day.
Analysis:
Determine the absorbance of the serum sample using a spectrophotometric cell of 1-cm path length at the wavelengths of absorbance at 576, 623, and 700 nm and using water as a blank. Calculate the concentration of hemoglobin in mg/dL:
(Abs576 × 115)  (Abs623 × 102)
(Abs623 × 102)  (Abs700 × 39.1)
(Abs700 × 39.1)
Acceptance criteria:
NMT 30 mg/dL
FBS FUNCTIONALITY TESTS
In the absence of a user-defined functionality assay, the following tests are suitable to determine the functionality of specific lots of FBS and to aid in the optimization of the growth conditions of mammalian cell cultures in the presence of FBS. For valid functionality confirmation independent of user-specific applications, tests are performed on the specified cell lines. For in-house validation of specialized cell culture applications, cell line(s) specific to those applications should be used and characterized. Use appropriate tissue culture vessels. Two tests described in this chapter are the Growth-Promotion Curve and the Clonal Assay. The decision about which type of test or the number of tests to be performed to assess suitability of a specific lot of FBS depends on the type of cell line used. For adherent cell lines, the number of colonies at the end of the culturing period represents a good assessment of the capacity of these cells, at low concentration, to grow in the presence of a specific lot of FBS. For cell lines growing in suspension cultures, the optimum growth kinetics is measured by counting viable cells after 7 days of culture.
Cell lines:
Five cell lines are recommended for use:
The functionality tests described are to be performed on three cell lines, two of which are drawn from the five recommended cell lines and the third of which is the cell line relevant to the user’s application. Cell lines are cultured with specific media as recommended by ATCC.
- HFL1 (ATCC CCL-153) normal lung, fibroblast
- Mv1 Lu (ATCC CCL-64) mink lung, epithelial
- HL-60 (ATCC CCL-240) peripheral blood promyeloblast, suspension
- VERO (ATCC CCL-81) monkey kidney fibroblast
- CHO (CCL-61) Chinese hamster ovary
Materials
— Suitable growth vessel/container
— Biological Safety Cabinet Class II, Type A
— Cell counter/hemacytometer
— Inverted microscope with digital camera accessory
— Tissue culture vessels: T25 cm2
Preparation of cells for assays:
Quick-thaw a vial in a 37 water bath, and determine cell count and viability. Prepare multiple cultures from each cell line in serum-supplemented growth medium. Incubate the cultures at 37
water bath, and determine cell count and viability. Prepare multiple cultures from each cell line in serum-supplemented growth medium. Incubate the cultures at 37 following instructions provided by ATCC for each of the cell lines used for the test. Examine the prepared cultures under a microscope to ensure uniform, near-confluent monolayers or suspensions. Expand cells until there are enough for assay (about 1 × 107 total cells; >90% viability).
following instructions provided by ATCC for each of the cell lines used for the test. Examine the prepared cultures under a microscope to ensure uniform, near-confluent monolayers or suspensions. Expand cells until there are enough for assay (about 1 × 107 total cells; >90% viability).
Harvesting of cultures
- Remove and discard the growth medium, and then rinse each culture with media lacking FBS.
-
For adherent cells, add 1 mL of Trypsin/EDTA for a few minutes for cells to disperse. Incubate at 37
 , if necessary. Neutralize with 1 mL culture medium containing at least 10% FBS.
, if necessary. Neutralize with 1 mL culture medium containing at least 10% FBS.
- Spin down the cells in a centrifuge. Aspirate off wash media, and resuspend cells in an appropriate volume for seeding.
Seeding of cells
-
On day 0: For the three cell lines to be tested prepare multiple cultures using seeding densities that range between 2 × 103 and 2 × 104 viable cells/mL. (Different inocula are chosen initially to determine optimum growth conditions. Once the appropriate inoculum is chosen, that condition is used to propagate the cells.) Following are the recommended seeding densities:
Low seeding density: 2 × 103 viable cells/mL
Mid seeding density: 6 × 103 viable cells/mL
High seeding density: 2 × 104 viable cells/mL - Prepare cultures in triplicate for at least five time points (in days or hours according to the cell line), to determine the seeding density that will yield the optimal growth conditions for each cell line used.
-
Incubate the cultures at 37
 in a humidified incubator saturated with 5% CO2.
in a humidified incubator saturated with 5% CO2.
- For each time point of measurement (days 0, 1, 2, 3, 4, and 7), take a photograph of each culture, in triplicate, for both the FBS test material and the USP Fetal Bovine Serum RS at each of the three concentrations for each cell line, and record the percentage of confluency for each of the conditions. [Note—Perform this step before trypsinization and cell counting. ]
- Harvest the cells from the three different seeding density cultures for each specific time point. For adherent cultures, harvest cells as described above.
- Perform and record total cell count and viability for each of the nine cultures for the FBS test and the USP Fetal Bovine Serum RS for each cell line using an appropriate cell counter or hemacytometer. [Notes—The schedule for counting may have to be changed for fast-growing cell lines or large cells that would become confluent before day 7 and/or for slow-growing lines that need to be in culture 8–10 days before reaching a plateau. Some adherent cell lines will never reach confluency. ]
Growth-Promotion Curve
Measurements of cell proliferation rates often are used to determine the response of cells to exogenous stimuli. Quantitative assessment of cell growth conditions is an important factor in monitoring consistency of culture conditions. The optimal cell concentration range for subculturing, optimum inoculum, and doubling time are parameters that can be quantified and trended. Information about the growth kinetics of a culture is critical in the design of cell-based experiments. Cultures vary significantly in their growth properties from lag phase, log phase, and stationary phase. Document the growth characteristics of the culture during the three growth stages to determine population doubling time and cell cycle time. Cells that have entered the stationary phase may demonstrate reduced growth potential and change in morphology. Cells may become polarized and may secrete more extracellular matrix, making them difficult to remove from the substratum. Cells at the end of the log phase give the highest yield and greatest reproducibility.
Reagents
— Growth media without FBS
— FBS test samples
— Growth medium + 10% FBS
— Trypsin/EDTA solution (0.25%/0.53 mM) in Hank’s Balanced Salt Solution (HBSS)
Analysis:
Once the cells have reached the end of the log phase, subculture the cells for the test. Follow the procedure described under Seeding Cells and prepare multiple cultures for the USP Fetal Bovine Serum RS, and test FBS for different cell lines at three seeding densities for which at least one growth curve displays a lag phase, log phase, and stationary phase and for which the log phase is linear at three or more time points.
Viable cell counts are determined on days 0, 1, 2, 3, 4, and 7.
Calculation and Data analysis:
Calculate the mean viable count [cells/cm2 (adherent) or cells/mL (suspension)] and the mean percent viability for each data point. Plot the data on a semi-log scale graph with the viable count on the log scale on the y-axis and days (or hours) in culture on an arithmetic scale on the x-axis. Estimate the doubling time using a growth curve that is linear over three or more time points.
Acceptance criteria:
The R2 value of the line should be equal to or greater than 0.98 in order to support calculation of a valid doubling time. The doubling time of the test sample should be no less than 90% of the doubling time of USP Fetal Bovine Serum RS.
Clonal Assay
This assay is designed to assess the optimal growth for adherent cell lines. Plating efficiency or colony formation at low cell density is a preferred method for analyzing the proliferative capacity and survival of single cells under optimal growth conditions. This is a very sensitive test and is often used for assessing the quality of serum lots. This technique reveals differences in the growth rate within the cell population and is capable of distinguishing between changes in growth rate (colony size) and cell survival (colony number). Because of the heterogeneous cell population of some cell cultures, remember that cells grow differently as isolated colonies at low densities. Consequently, few cells survive even under ideal conditions because all cell interaction is lost. Cloning is a survival assay that is also used for optimizing growth conditions (selection of medium and serum). If it can be confirmed that a single colony arose from a single cell, then cloning efficiency can be determined.
Reagents
— Growth medium + 10% FBS (test serum)—Eagle minimum essential medium (EMEM) with 2 mM l-glutamine and Earle’s BSS adjusted to contain 1.5 g/L sodium carbonate, 0.1 mM nonessential amino acids, and sodium pyruvate containing 100 U/mL penicillin and 100 g/mL streptomycin plus 10% FBS.
— Trypsin/EDTA solution (0.25%/0.53 mM) in HBSS.
— Dulbecco’s Phosphate Buffer Saline without calcium or magnesium.
— Carbol Fuchsin–Methylene Blue Solution—Mix 20 g carbol fuchsin stock in 2 L methanol and stir for 10 min (1% carbol fuchsin). Mix 50 g methylene blue in 5 L methanol, and stir for 10 min (1% methylene blue). Prepare Carbol Fuschin–Methylene Blue working solution by mixing methylene blue, methanol, and carbol fuchsin in a ratio of 3:2:1. Mix for 20 min and filter through four folds of cheesecloth in a funnel. Aliquot and store in brown glass bottles at 15 to 25
to 25 .
.
Sample:
Multiple lots of FBS are used for this assay. For each lot of serum to be tested, add 20 mL of FBS to 180 mL of EMEM, and use the same sample for the entire test. Sterilize using 0.22-m low protein binding filter units. Store growth medium at 4 until ready to use.
until ready to use.
Cell preparation:
This test is only for adherent cultures and is performed with the adherent cell lines described under Cell Lines (HFL1 and Mv 1 Lu). One week before testing serum, expand the cell lines as described under Seeding of Cells, change the medium every 2–3 days, and subculture the cells when they are about 90% confluent. Determine the cell count and viability (viability should be >90%) before performing the assay. Harvest cells as described under Harvesting of Cultures, wash twice, and resuspend cells in basal EMEM.
Analysis:
The procedure involves plating single-cell suspension at low densities (2–50 cells/cm2) from which discrete colonies will form. At the end of the assay, fix, stain, and count the number of colonies as directed below.
- For each cell line label ten 60-mm × 15-mm tissue culture dishes for each serum lot that will be tested. Label the side of the lower half of each dish, including controls.
- Transfer 5 mL of medium containing 10% of the appropriate test serum (10 replicates). Add 400 cells per culture dish (aim for a cell concentration of about 800 cells/mL).
-
Incubate for 10–14 days at 37
 in a humidified incubator saturated with 5% CO2.
in a humidified incubator saturated with 5% CO2.
- Remove the supernatant and add enough Carbol Fuchsin–Methylene Blue Solution to cover each of the culture dishes for 10 min.
- Remove the stain; rinse the culture dishes with several changes of distilled water; invert the dishes on paper towels; and allow to dry.
- Count and record (1) the number of colonies and (2) the total surface of stained colonies (mm2). Calculate means and standard deviations.
Acceptance criteria:
Percent plating efficiency is expressed by counting the number of colonies in a defined area divided by the number of cells seeded multiplied by 100. Compare results between lots of FBS, and select a serum lot that is good for various types of cells and optimal for a specific cell culture application.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| General Chapter | Fouad Atouf, Ph.D.
Senior Scientific Liaison 1-301-816-8365 |
(GCBA2010) General Chapters - Biological Analysis |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
|
| Radhakrishna S Tirumalai, Ph.D.
Principal Scientific Liaison 1-301-816-8339 |
(GCM2010) General Chapters - Microbiology | |
| Radhakrishna S Tirumalai, Ph.D.
Principal Scientific Liaison 1-301-816-8339 |
(GCM2010) General Chapters - Microbiology |
USP35–NF30 Page 98
Pharmacopeial Forum: Volume No. 35(5) Page 1219