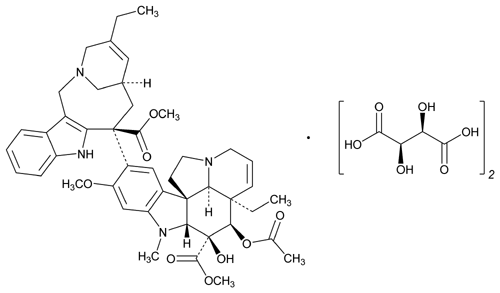
Vinorelbine Tartrate
» Vinorelbine Tartrate contains not less than 98.0 percent and not more than 102.0 percent of C45H54N4O8·2C4H6O6, calculated on the anhydrous basis.
Caution—Vinorelbine Tartrate is cytotoxic. Great care should be taken to prevent inhaling particles and exposing the skin to it.
Packaging and storage—
Preserve in tight, light-resistant containers. Store in a freezer.
Clarity of solution—
Dissolve an amount of Vinorelbine Tartrate, equivalent to 100.0 mg of anhydrous vinorelbine, in 10 mL of water: the solution is clear.
Color of solution—
The absorbance of the solution prepared under Clarity of solution, determined in a 1-cm cell at 420 nm in a suitable spectrophotometer, using water as the blank, is not more than 0.03.
Identification—
A:
Infrared Absorption  197K
197K —
—
Test specimen—
Dissolve 10 mg in 5 mL of water, add 0.5 mL of 5 N sodium hydroxide, and extract with 5 mL of methylene chloride. Filter the organic extract through anhydrous sodium sulfate, and evaporate the organic extract to a volume of about 0.5 mL.
B:
The retention time of the major peak in the chromatogram of the Test solution corresponds to that in the chromatogram of the Diluted standard solution, as obtained in the test for Related compounds.
C:
To 0.1 mL of a solution containing the equivalent of about 15 mg of tartaric acid per mL, add 0.1 mL of a 100 g per L solution of potassium bromide, 0.1 mL of a 20 g per L solution of resorcinol, and 3 mL of sulfuric acid. Heat on a hot water bath for 5 to 10 minutes until a dark blue color develops. Allow to cool, and pour the solution into water. The color changes to red (presence of tartrate).
pH  791
791 :
:
 between 3.3 and 3.8, in a solution (10 mg per mL).
between 3.3 and 3.8, in a solution (10 mg per mL).
Water, Method Ia  921
921 :
:
 not more than 4.0%.
not more than 4.0%.
Residue on ignition  281
281 :
:
 not more than 0.1%.
not more than 0.1%.
Related compounds—
Phosphate buffer, Mobile phase, System suitability solution, and Chromatographic system—
Proceed as directed in the Assay.
Standard solution—
Use the Standard preparation, prepared as directed in the Assay.
Diluted standard solution—
Transfer 1.0 mL of the Standard solution to a 50-mL volumetric flask, and dilute with Mobile phase to volume. Pipet 1.0 mL of this solution into a 100-mL volumetric flask, and dilute with Mobile phase to volume.
Test solution—
Use the Assay preparation.
Procedure—
Separately inject equal volumes (about 20 µL) of the Test solution and the Diluted standard solution into the chromatograph, record the chromatograms, and measure the areas for the major peaks. Record the chromatograms for three times the retention time of the vinorelbine peak. Disregard any peaks with an area less than or equal to one-half of the area of the peak obtained for vinorelbine in the Diluted standard solution. Calculate the percentage of each impurity in the portion of Vinorelbine Tartrate taken by the formula:
100(ri / rs)
in which ri is the peak response for each impurity obtained from the Test solution; and rs is the sum of the responses of all the peaks: not more than 0.3% of the photodegradation product is found; not more than 0.2% of any individual impurity or coeluted impurities comprising an individual peak is found; and not more than 0.7% of total impurities, excluding the photodegradation product, is found.
Assay—
Phosphate buffer—
Dissolve 6.9 g of monobasic sodium phosphate in 900 mL of water. Adjust with phosphoric acid to a pH of 4.2, dilute with water to 1000 mL, and mix.
Mobile phase—
Dissolve 1.22 g of sodium 1-decanesulfonate in 620 mL of methanol. Add 380 mL of Phosphate buffer, mix, filter, and degas. Make adjustments if necessary (see System Suitability under Chromatography  621
621 ).
).
System suitability solution—
Dissolve accurately weighed quantities of USP Vinorelbine Tartrate RS and USP Vinorelbine Related Compound A RS in water, and dilute quantitatively, and stepwise if necessary, with water to obtain a solution having known concentrations of about 1.4 mg per mL and 0.01 mg per mL, respectively. Expose a portion of this solution in a suitable xenon lamp apparatus capable of supplying a dose of 1600 KJ/m2 between 310 and 800 nm at a power of 500 W/m2 for about 1 hour, in order to generate an additional degradation product (3¢,4¢,7,8-tetradehydro-3,4¢-dideoxy-3,6-epoxy-6,7-dihydro-C¢-norvincaleukoblastine) having a relative retention time of about 0.8.
Standard preparation—
Dissolve an accurately weighed quantity of USP Vinorelbine Tartrate RS in Mobile phase to obtain a solution having a known concentration of about 1.4 mg per mL.
Assay preparation—
Dissolve an accurately weighed quantity of Vinorelbine Tartrate in Mobile phase to obtain a solution having a known concentration of about 1.4 mg per mL.
Chromatographic system (see Chromatography  621
621 )—
The liquid chromatograph is equipped with a 267-nm detector and a 3.9-mm × 15-cm column that contains 5-µm packing L1. The column temperature is maintained at 40
)—
The liquid chromatograph is equipped with a 267-nm detector and a 3.9-mm × 15-cm column that contains 5-µm packing L1. The column temperature is maintained at 40 . The flow rate is about 1.0 mL per minute. Chromatograph the System suitability solution, and record the peak responses as directed for Procedure: the relative retention, r, between vinorelbine tartrate and vinorelbine related compound A is not less than 1.1. Chromatograph the Standard preparation, and record the peak responses as directed for Procedure: the relative standard deviation for replicate injections is not more than 2.0%.
[note—For peak identification purposes, the relative retention times are about 0.8 for the photodegradation product, 1.0 for vinorelbine, and 1.2 for vinorelbine related compound A.]
. The flow rate is about 1.0 mL per minute. Chromatograph the System suitability solution, and record the peak responses as directed for Procedure: the relative retention, r, between vinorelbine tartrate and vinorelbine related compound A is not less than 1.1. Chromatograph the Standard preparation, and record the peak responses as directed for Procedure: the relative standard deviation for replicate injections is not more than 2.0%.
[note—For peak identification purposes, the relative retention times are about 0.8 for the photodegradation product, 1.0 for vinorelbine, and 1.2 for vinorelbine related compound A.]
Procedure—
Separately inject equal volumes (about 20 µL) of the Standard preparation and the Assay preparation into the chromatograph, record the chromatograms, and measure the responses for the vinorelbine tartrate peaks. Calculate the quantity, in percentage, of C45H54N4O8·2C4H6O6 in the portion of Vinorelbine Tartrate taken by the formula:
100(CS / CU)(rU/rS)
in which CS is the concentration, in mg per mL, of USP Vinorelbine Tartrate RS in the Standard preparation; CU is the concentration of Vinorelbine Tartrate in the Assay preparation; and rU and rS are the peak responses obtained from the Assay preparation and the Standard preparation, respectively.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
Chromatographic Column—
| Topic/Question | Contact | Expert Committee |
| Monograph | Feiwen Mao, M.S.
Scientist 1-301-816-8320 |
(MDOOD05) Monograph Development-Ophthalmics Oncologics and Dermatologicals |
| Reference Standards | Lili Wang, Technical Services Scientist 1-301-816-8129 RSTech@usp.org |
USP32–NF27 Page 3863
Pharmacopeial Forum: Volume No. 32(5) Page 1471
Chromatographic columns text is not derived from, and not part of, USP 32 or NF 27.