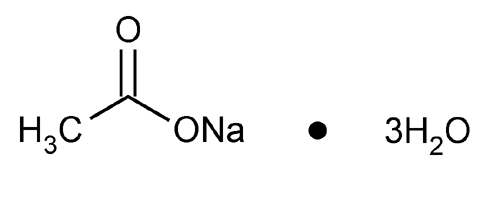
Sodium Acetate
Acetic acid, sodium salt, trihydrate.
Sodium acetate trihydrate
Anhydrous 82.03
» Sodium Acetate contains three molecules of water of hydration, or is anhydrous. It contains not less than 99.0 percent and not more than 101.0 percent of C2H3NaO2, calculated on the dried basis.
Packaging and storage—
Preserve in tight containers.
Labeling—
Label it to indicate whether it is the trihydrate or is anhydrous. Where Sodium Acetate is intended for use in hemodialysis, it is so labeled.
pH  791
791 :
between 7.5 and 9.2, in a solution in carbon dioxide-free water containing the equivalent of 30 mg of anhydrous sodium acetate per mL.
:
between 7.5 and 9.2, in a solution in carbon dioxide-free water containing the equivalent of 30 mg of anhydrous sodium acetate per mL.
Loss on drying  731
731 —
Dry at 120
—
Dry at 120 to constant weight: the hydrous form loses between 38.0% and 41.0% of its weight, and the anhydrous form loses not more than 1.0% of its weight.
to constant weight: the hydrous form loses between 38.0% and 41.0% of its weight, and the anhydrous form loses not more than 1.0% of its weight.
Insoluble matter—
Dissolve the equivalent of 20 g of anhydrous sodium acetate in 150 mL of water, heat to boiling, and digest in a covered beaker on a steam bath for 1 hour. Filter through a tared filtering crucible, wash thoroughly, and dry at 105 : the weight of the residue does not exceed 10 mg (0.05%).
: the weight of the residue does not exceed 10 mg (0.05%).
Chloride  221
221 —
A portion equivalent to 1.0 g of anhydrous sodium acetate shows no more chloride than corresponds to 0.50 mL of 0.020 N hydrochloric acid (0.035%).
—
A portion equivalent to 1.0 g of anhydrous sodium acetate shows no more chloride than corresponds to 0.50 mL of 0.020 N hydrochloric acid (0.035%).
Sulfate  221
221 —
A portion equivalent to 10 g of anhydrous sodium acetate shows no more sulfate than corresponds to 0.50 mL of 0.020 N sulfuric acid (0.005%).
—
A portion equivalent to 10 g of anhydrous sodium acetate shows no more sulfate than corresponds to 0.50 mL of 0.020 N sulfuric acid (0.005%).
Calcium and magnesium—
To 20 mL of a solution containing the equivalent of 10 mg of anhydrous sodium acetate per mL add 2 mL each of 6 N ammonium hydroxide, ammonium oxalate TS, and dibasic sodium phosphate TS: no turbidity is produced within 5 minutes.
Potassium—
Dissolve the equivalent of 3 g of anhydrous sodium acetate in 5 mL of water, add 1 N acetic acid dropwise until the solution is slightly acidic, and then add 5 drops of sodium cobaltinitrite TS: no precipitate is formed.
Aluminum  206
206 (where it is labeled as intended for use in hemodialysis)—
Proceed as directed using 10.0 g of Sodium Acetate to prepare the Test Preparation: the limit is 0.2 µg per g.
(where it is labeled as intended for use in hemodialysis)—
Proceed as directed using 10.0 g of Sodium Acetate to prepare the Test Preparation: the limit is 0.2 µg per g.
Heavy metals, Method I  231
231 —
Dissolve a portion equivalent to 4.2 g of C2H3NaO2 in enough water to make 50 mL of stock solution. Transfer 12 mL of this stock solution to a 50-mL color-comparison tube (Test Preparation). Transfer 11 mL of the stock solution to a second color-comparison tube containing 1.0 mL of Standard Lead Solution (Monitor Preparation). Transfer 1.0 mL of Standard Lead Solution and 11 mL of water to a third color-comparison tube (Standard Preparation). Proceed as directed for Procedure, omitting the dilution to 50 mL: the limit is 0.001%.
—
Dissolve a portion equivalent to 4.2 g of C2H3NaO2 in enough water to make 50 mL of stock solution. Transfer 12 mL of this stock solution to a 50-mL color-comparison tube (Test Preparation). Transfer 11 mL of the stock solution to a second color-comparison tube containing 1.0 mL of Standard Lead Solution (Monitor Preparation). Transfer 1.0 mL of Standard Lead Solution and 11 mL of water to a third color-comparison tube (Standard Preparation). Proceed as directed for Procedure, omitting the dilution to 50 mL: the limit is 0.001%.
Assay—
Weigh accurately the equivalent of about 200 mg of anhydrous sodium acetate, and dissolve in 25 mL of glacial acetic acid, warming gently if necessary to effect complete solution. Add 2 drops of p-naphtholbenzein TS, and titrate with 0.1 N perchloric acid VS. Perform a blank determination, and make any necessary correction. Each mL of 0.1 N perchloric acid is equivalent to 8.203 mg of C2H3NaO2.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
Chromatographic Column—
| Topic/Question | Contact | Expert Committee |
| Monograph | Daniel K. Bempong, Ph.D.
Senior Scientist 1-301-816-8143 |
(MDPS05) Monograph Development-Pulmonary and Steroids |
USP32–NF27 Page 3561
Pharmacopeial Forum: Volume No. 29(5) Page 1576
Chromatographic columns text is not derived from, and not part of, USP 32 or NF 27.