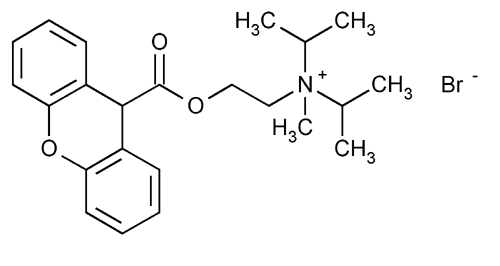
Propantheline Bromide
2-Propanaminium, N-methyl-N-(1-methylethyl)-N-[2-[(9H-xanthen-9-ylcarbonyl)oxy]ethyl]-, bromide.
(2-Hydroxyethyl)diisopropylmethylammonium bromide xanthene-9-carboxylate
» Propantheline Bromide contains not less than 98.0 percent and not more than 102.0 percent of C23H30BrNO3, calculated on the dried basis.
Packaging and storage—
Preserve in well-closed containers.
USP Reference standards  11
11 —
—
USP Xanthanoic Acid RS.
USP Xanthone RS.
USP Propantheline Bromide RS.
USP Propantheline Bromide Related Compound A RS .
.
USP Xanthanoic Acid RS.
USP Xanthone RS.
USP Propantheline Bromide RS.
USP Propantheline Bromide Related Compound A RS
 .
.
Identification—
A:
Prepare 3 mL of a solution in chloroform having a concentration of about 6 mg per mL, and reserve a 1-mL portion for Identification test B. In a well-ventilated hood, apply 2 mL of this solution dropwise to a salt plate while continuously evaporating the solvent with the aid of an IR heat lamp and a current of dry air. Heat the residue at 105 for 15 minutes: the IR absorption spectrum of the residue on the single salt plate exhibits maxima only at the same wavelengths as that of a similar preparation of USP Propantheline Bromide RS, treated in the same manner.
for 15 minutes: the IR absorption spectrum of the residue on the single salt plate exhibits maxima only at the same wavelengths as that of a similar preparation of USP Propantheline Bromide RS, treated in the same manner.
B:
Apply 5 µL of the chloroform solution retained from Identification test A and 5 µL of a Standard solution of USP Propantheline Bromide RS in chloroform containing 6 mg per mL to a suitable thin-layer chromatographic plate (see Chromatography  621
621 ) coated with a 0.25-mm layer of chromatographic silica gel. Develop the chromatogram in a solvent system consisting of a mixture of 1 N hydrochloric acid and acetone (1:1) until the solvent front has moved about three-fourths of the length of the plate. Remove the plate from the developing chamber, mark the solvent front, and dry at 105
) coated with a 0.25-mm layer of chromatographic silica gel. Develop the chromatogram in a solvent system consisting of a mixture of 1 N hydrochloric acid and acetone (1:1) until the solvent front has moved about three-fourths of the length of the plate. Remove the plate from the developing chamber, mark the solvent front, and dry at 105 for 5 minutes. Spray the plate with potassium-bismuth iodide TS, and heat at 105
for 5 minutes. Spray the plate with potassium-bismuth iodide TS, and heat at 105 for 5 minutes: the RF value of the principal spot obtained from the test solution corresponds to that obtained from the Standard solution.
for 5 minutes: the RF value of the principal spot obtained from the test solution corresponds to that obtained from the Standard solution.
C:
To 5 mL of a solution (1 in 100) add 2 mL of 2 N nitric acid: this solution responds to the tests for Bromide  191
191 , except that in the test that liberates bromine, the chloroform layer may be yellow.
, except that in the test that liberates bromine, the chloroform layer may be yellow.
Loss on drying  731
731 —
Dry it at 105
—
Dry it at 105 for 4 hours: it loses not more than 0.5% of its weight.
for 4 hours: it loses not more than 0.5% of its weight.
Residue on ignition  281
281 :
not more than 0.1%.
:
not more than 0.1%.
Related compounds—
pH 3.5 buffer solution—
Dissolve 17.3 g of sodium dodecyl sulfate in 1000 mL of water containing 10 mL of phosphoric acid in a 2000-mL volumetric flask. Add 250 mL of 0.5 M sodium hydroxide and, while stirring, adjust with 0.5 M sodium hydroxide or dilute phosphoric acid (1 in 10) to a pH of 3.5 ± 0.05, dilute with water to volume, and mix.
Mobile phase—
Prepare a filtered and degassed mixture of acetonitrile and pH 3.5 buffer solution (55:45). Make adjustments if necessary (see System Suitability under Chromatography  621
621 ).
).
Standard solution—
Dissolve accurately weighed quantities of USP Propantheline Bromide Related Compound A RS, USP Xanthanoic Acid RS, and USP Xanthone RS in Mobile phase, and dilute quantitatively, and stepwise if necessary, with Mobile phase to obtain a solution having a known concentration of about 6.0 µg of propantheline bromide related compound A per mL, and about 1.5 µg each of xanthanoic acid and xanthone per mL.
Test solution—
Transfer about 60 mg of Propantheline Bromide, accurately weighed, to a 200-mL volumetric flask, dissolve in Mobile phase, dilute with Mobile phase to volume, and mix.
Chromatographic system (see Chromatography  621
621 )—
The liquid chromatograph is equipped with a 254-nm detector and a 4.6-mm × 25-cm column that contains packing L7. The flow rate is about 2.0 mL per minute. Chromatograph the Standard solution, and record peak responses as directed for Procedure: the resolution, R, between the least resolved peaks is not less than 1.2; and the relative standard deviation for replicate injections of the Standard solution is not more than 6.0% for each component.
)—
The liquid chromatograph is equipped with a 254-nm detector and a 4.6-mm × 25-cm column that contains packing L7. The flow rate is about 2.0 mL per minute. Chromatograph the Standard solution, and record peak responses as directed for Procedure: the resolution, R, between the least resolved peaks is not less than 1.2; and the relative standard deviation for replicate injections of the Standard solution is not more than 6.0% for each component.
Procedure—
Separately inject equal volumes (about 50 µL) of the Standard solution and the Test solution into the chromatograph, record the chromatograms for a total time of not less than 1.5 times the retention time of the propantheline bromide peak, and measure the response for each peak, except the peaks at or before the void volume. Calculate the percentage of xanthanoic acid, xanthone, and propantheline bromide related compound A greater than or equal to 0.1% in the portion of Propantheline Bromide taken by the formula:
20C/W(rU / rS)
in which C is the concentration, in µg, of xanthanoic acid, xanthone, or propantheline bromide related compound A per mL of the Standard solution; W is the weight, in mg, of Propantheline Bromide taken; and rU and rS are the related compound peak responses obtained from the Test solution and the Standard solution, respectively: not more than 2.0% of propantheline bromide related compound A and 0.5% each of xanthone and xanthanoic acid is found. Calculate the percentage of all unknown impurities greater than or equal to 0.1% by the formula:
100ri / rt
in which ri is the response of the unknown impurity peak; and rt is the sum of the responses of all the measured peaks observed in the chromatogram: the sum total of all known and unknown impurities is not more than 3.0%.
Bromide content—
Weigh accurately about 500 mg, and dissolve in 40 mL of water. Add 10 mL of glacial acetic acid and 40 mL of methanol, then add eosin Y TS, and titrate with 0.1 N silver nitrate VS. Each mL of 0.1 N silver nitrate is equivalent to 7.990 mg of Br. Not less than 17.5% and not more than 18.2% of Br, calculated on the dried basis, is found.
Assay—
Dissolve about 600 mg of Propantheline Bromide, accurately weighed, in a mixture of 20 mL of glacial acetic acid and 15 mL of mercuric acetate TS, warming slightly if necessary to effect solution. Cool to room temperature, and titrate with 0.1 N perchloric acid VS, determining the endpoint potentiometrically. Perform a blank determination, and make any necessary correction. Each mL of 0.1 N perchloric acid is equivalent to 44.84 mg of C23H30BrNO3.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
Chromatographic Column—
| Topic/Question | Contact | Expert Committee |
| Monograph | Elena Gonikberg, Ph.D.
Senior Scientist 1-301-816-8251 |
(MDGRE05) Monograph Development-Gastrointestinal Renal and Endocrine |
| Reference Standards | Lili Wang, Technical Services Scientist 1-301-816-8129 RSTech@usp.org |
USP32–NF27 Page 3409
Pharmacopeial Forum: Volume No. 29(2) Page 430
Chromatographic columns text is not derived from, and not part of, USP 32 or NF 27.