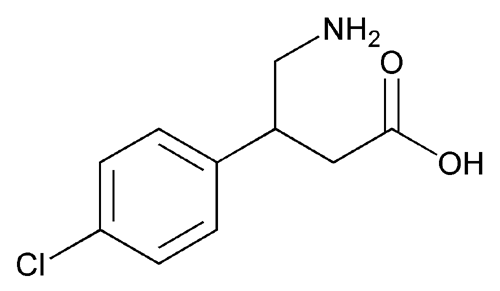
Baclofen
Butanoic acid, 4-amino-3-(4-chlorophenyl)-.
» Baclofen contains not less than 99.0 percent and not more than 101.0 percent of C10H12ClNO2, calculated on the anhydrous basis.
Packaging and storage—
Preserve in tight containers.
Water, Method I  921
921 :
not more than 3.0%.
:
not more than 3.0%.
Residue on ignition  281
281 :
not more than 0.3%.
:
not more than 0.3%.
Heavy metals, Method II  231
231 :
0.001%.
:
0.001%.
Related compounds—
[Caution: Avoid contact with o-tolidine when performing this test, and conduct the test in a well-ventilated hood.
]
Detection reagent—
Dissolve 200 mg of o-tolidine in 2.0 mL of glacial acetic acid with the aid of a hot water bath. Dilute with water to 100.0 mL, and filter. Mix one volume of this solution with an equal volume of potassium iodide solution (0.83 in 100).
Diluting solution—
Prepare a mixture of alcohol and glacial acetic acid (4:1).
Standard preparations—
Dissolve USP Baclofen RS in Diluting solution to obtain a Standard stock solution having a known concentration of 0.1 mg per mL. Dilute portions of this Standard stock solution quantitatively with Diluting solution to obtain solutions having concentrations of 0.05 mg per mL (Standard preparation A) and 0.03 mg per mL (Standard preparation B), respectively.
Identification preparation—
Dissolve USP Baclofen Related Compound A RS in Diluting solution to obtain a solution having a known concentration of 0.1 mg per mL.
Test preparation—
Prepare a solution in Diluting solution containing 10.0 mg of Baclofen per mL.
Procedure—
Apply separately 10 µL of the Test preparation, Identification preparation, Standard preparation A, and Standard preparation B to a suitable thin-layer chromatographic plate (see Chromatography  621
621 , coated with a 0.25-mm layer of chromatographic silica gel mixture. Place the plate in a chromatographic chamber, and develop the chromatograms in a solvent system consisting of a mixture of butyl alcohol, glacial acetic acid, and water (4:1:1) until the solvent front has moved about three-fourths of the length of the plate. Remove the plate from the developing chamber, and dry under a current of warm air. Transfer the dry plate to another chromatographic chamber containing a beaker with 1 g of potassium permanganate. Add 10 mL of dilute hydrochloric acid (4 in 10) to the beaker. Cover the chamber, and allow the chamber to become saturated with chlorine gas. Expose the plate to the chlorine gas for 8 minutes. Remove the plate and expose to the air for 2 minutes, then spray with freshly prepared Detection reagent: the intensity of the secondary spot from the Test preparation, corresponding in RF value to the principal spot from the Identification preparation, is not greater than that of the principal spot from the Identification preparation (1.0%), and no other secondary spot from the Test preparation is greater in intensity than the principal spot from Standard preparation A (0.5%). The sum of all secondary spots from the Test preparation is not more than 2.0%.
, coated with a 0.25-mm layer of chromatographic silica gel mixture. Place the plate in a chromatographic chamber, and develop the chromatograms in a solvent system consisting of a mixture of butyl alcohol, glacial acetic acid, and water (4:1:1) until the solvent front has moved about three-fourths of the length of the plate. Remove the plate from the developing chamber, and dry under a current of warm air. Transfer the dry plate to another chromatographic chamber containing a beaker with 1 g of potassium permanganate. Add 10 mL of dilute hydrochloric acid (4 in 10) to the beaker. Cover the chamber, and allow the chamber to become saturated with chlorine gas. Expose the plate to the chlorine gas for 8 minutes. Remove the plate and expose to the air for 2 minutes, then spray with freshly prepared Detection reagent: the intensity of the secondary spot from the Test preparation, corresponding in RF value to the principal spot from the Identification preparation, is not greater than that of the principal spot from the Identification preparation (1.0%), and no other secondary spot from the Test preparation is greater in intensity than the principal spot from Standard preparation A (0.5%). The sum of all secondary spots from the Test preparation is not more than 2.0%.
Assay—
Dissolve about 200 mg of Baclofen, accurately weighed, in a suitable volume of glacial acetic acid, sufficient to immerse the electrodes. Titrate with 0.1 N perchloric acid VS, determining the endpoint potentiometrically, using a glass electrode and a calomel electrode containing a saturated solution of lithium chloride in glacial acetic acid (see Titrimetry  541
541 ). Perform a blank determination, and make any necessary correction. Each mL of 0.1 N perchloric acid is equivalent to 21.37 mg of C10H12ClNO2.
). Perform a blank determination, and make any necessary correction. Each mL of 0.1 N perchloric acid is equivalent to 21.37 mg of C10H12ClNO2.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
Chromatographic Column—
| Topic/Question | Contact | Expert Committee |
| Monograph | Ravi Ravichandran, Ph.D.
Senior Scientist 1-301-816-8330 |
(MDPP05) Monograph Development-Psychiatrics and Psychoactives |
| Reference Standards | Lili Wang, Technical Services Scientist 1-301-816-8129 RSTech@usp.org |
USP32–NF27 Page 1625
Pharmacopeial Forum: Volume No. 33(2) Page 211
Chromatographic columns text is not derived from, and not part of, USP 32 or NF 27.