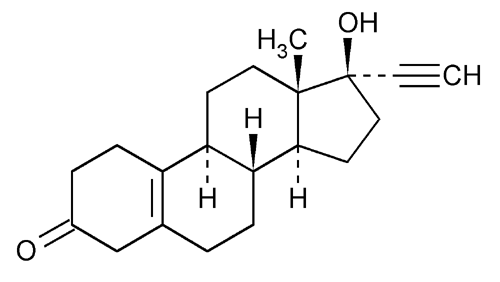
Norethynodrel
19-Norpregn-5(10)-en-20-yn-3-one, 17-hydroxy-, (17
17-Hydroxy-19-nor-17
» Norethynodrel contains not less than 97.0 percent and not more than 101.0 percent of C20H26O2.
Packaging and storage—
Preserve in well-closed containers.
Limit of ethynyl group—
Dissolve 200 mg in about 40 mL of tetrahydrofuran. Add 10 mL of silver nitrate solution (1 in 10), and titrate with 0.1 N sodium hydroxide VS, using either a glass-calomel or a silver-silver chloride electrode system with potassium nitrate filling solution. Each mL of 0.1 N sodium hydroxide is equivalent to 2.503 mg of ethynyl group (–C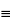 CH). Not less than 8.18% and not more than 8.43% of ethynyl group is found.
CH). Not less than 8.18% and not more than 8.43% of ethynyl group is found.
Limit of norethindrone—
Test preparation—
Prepare a solution of Norethynodrel in chloroform containing 10 mg per mL.
Standard solution—
Prepare a solution of USP Norethindrone RS in chloroform to contain 1 mg per mL. Dilute 2 mL of the solution with chloroform to 10 mL.
Procedure—
Apply 10-µL volumes of the Test preparation and the Standard solution (see Chromatography  621
621 ) to a thin-layer chromatographic plate coated with a 0.25-mm layer of chromatographic silica gel mixture, and allow not more than 5 minutes between spotting the plate and starting development of the chromatogram. Place the plate in a suitable chromatographic chamber previously equilibrated with a mixture of cyclohexane, ethyl acetate, and methanol (60:40:2), and allow the solvent front to move 15 cm. Spray the plate with dilute sulfuric acid (1 in 2), heat the plate at 105
) to a thin-layer chromatographic plate coated with a 0.25-mm layer of chromatographic silica gel mixture, and allow not more than 5 minutes between spotting the plate and starting development of the chromatogram. Place the plate in a suitable chromatographic chamber previously equilibrated with a mixture of cyclohexane, ethyl acetate, and methanol (60:40:2), and allow the solvent front to move 15 cm. Spray the plate with dilute sulfuric acid (1 in 2), heat the plate at 105 for 5 minutes, and view under long-wavelength UV light. Locate any norethindrone impurity in the Test preparation by comparison with the RF value from the Standard solution. If present, the norethindrone spot from the Test preparation is not larger or more intense than the spot from the Standard solution (2.0%).
for 5 minutes, and view under long-wavelength UV light. Locate any norethindrone impurity in the Test preparation by comparison with the RF value from the Standard solution. If present, the norethindrone spot from the Test preparation is not larger or more intense than the spot from the Standard solution (2.0%).
Ordinary impurities  466
466 —
—
Test solution:
chloroform.
Standard solution:
chloroform.
Eluant:
ether.
Visualization:
5, followed by viewing under long-wavelength UV light.
Assay—
Standard preparation—
Dissolve a suitable quantity of USP Norethynodrel RS, accurately weighed, in methanol, and dilute quantitatively with methanol to obtain a solution having a known concentration of about 1 mg per mL.
Assay preparation—
Dissolve about 100 mg of Norethynodrel, accurately weighed, in methanol to make 100.0 mL, and mix.
Procedure—
Transfer 10.0 mL each of the Standard preparation and the Assay preparation to separate 100-mL volumetric flasks. To each flask add 40 mL of methanol, then add 5 mL of a mixture of 3 volumes of hydrochloric acid and 2 volumes of water, mix quickly, and allow to stand at a temperature of about 25 for 1 hour, accurately timed. Prior to the end of the 1-hour period, prepare blanks as follows. Add 1.0 mL each of the Standard preparation and the Assay preparation to separate 100-mL volumetric flasks, each containing a mixture of 50 mL of methanol and 2 mL of water, dilute each with methanol to volume, and mix. At the end of the 1-hour reaction period, dilute each of the acid-containing solutions with methanol to volume, and mix. Transfer 10.0 mL of each into separate 100-mL volumetric flasks, add 2 mL of water to each, dilute with methanol to volume, and mix. Concomitantly determine the absorbances of the solutions in 1-cm cells at the wavelength of maximum absorbance at about 240 nm, with a suitable spectrophotometer, relative to the corresponding blanks. Calculate the quantity, in mg, of C20H26O2 in the portion of Norethynodrel taken by the formula:
for 1 hour, accurately timed. Prior to the end of the 1-hour period, prepare blanks as follows. Add 1.0 mL each of the Standard preparation and the Assay preparation to separate 100-mL volumetric flasks, each containing a mixture of 50 mL of methanol and 2 mL of water, dilute each with methanol to volume, and mix. At the end of the 1-hour reaction period, dilute each of the acid-containing solutions with methanol to volume, and mix. Transfer 10.0 mL of each into separate 100-mL volumetric flasks, add 2 mL of water to each, dilute with methanol to volume, and mix. Concomitantly determine the absorbances of the solutions in 1-cm cells at the wavelength of maximum absorbance at about 240 nm, with a suitable spectrophotometer, relative to the corresponding blanks. Calculate the quantity, in mg, of C20H26O2 in the portion of Norethynodrel taken by the formula:
100C(AU / AS)
in which C is the concentration, in mg per mL, of USP Norethynodrel RS in the Standard preparation, and AU and AS are the absorbances of the solutions from the Assay preparation and the Standard preparation, respectively.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
Chromatographic Column—
| Topic/Question | Contact | Expert Committee |
| Monograph | Daniel K. Bempong, Ph.D.
Senior Scientist 1-301-816-8143 |
(MDPS05) Monograph Development-Pulmonary and Steroids |
| Reference Standards | Lili Wang, Technical Services Scientist 1-301-816-8129 RSTech@usp.org |
USP32–NF27 Page 3111
Chromatographic columns text is not derived from, and not part of, USP 32 or NF 27.