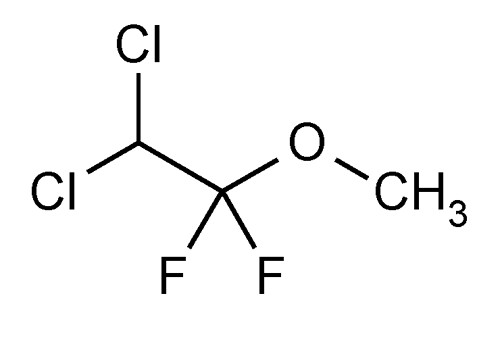
Methoxyflurane
Ethane, 2,2-dichloro-1,1-difluoro-1-methoxy-.
2,2-Dichloro-1,1-difluoroethyl methyl ether
» Methoxyflurane contains not less than 99.9 percent and not more than 100.0 percent of C3H4Cl2F2O. It may contain a suitable stabilizer.
Packaging and storage—
Preserve in tight, light-resistant containers, and avoid exposure to excessive heat.
Identification—
A:
The IR absorption spectrum of a 1 in 20 solution in chloroform exhibits maxima only at the same wavelengths as that of a similar solution of USP Methoxyflurane RS.
B:
To 1 mL in a test tube add 1 mL of sulfuric acid: the specimen forms a layer over the acid (distinction from halothane).
C:
Cautiously heat the contents of the test tube from Identification test B with agitation: the interface disappears, and hydrofluoric acid is evolved (distinction from chloroform, trichloroethylene, and halothane).
Specific gravity  841
841 :
between 1.420 and 1.425.
:
between 1.420 and 1.425.
Acidity—
Shake 25 mL with 25 mL of carbon dioxide-free water for 2 minutes, and allow the layers to separate. Add 1 drop of methyl red TS to the water extract, boil for 1 minute, and titrate with 0.010 N sodium hydroxide: not more than 0.50 mL of 0.010 N sodium hydroxide is required to produce a distinct yellow color.
Water, Method I  921
921 :
not more than 0.1%.
:
not more than 0.1%.
Limit of nonvolatile residue—
Allow 50 mL to evaporate at room temperature in a tared evaporating dish, and dry the residue at 105 for 1 hour: the weight of the residue does not exceed 1 mg.
for 1 hour: the weight of the residue does not exceed 1 mg.
Assay—
Inject a volume of Methoxyflurane (30 µL or less) into a suitable gas chromatograph (see Chromatography  621
621 ) equipped with a thermal conductivity detector. Under typical conditions, the instrument contains a 4-mm × 3-m stainless steel column packed with liquid phase G11 on support S1A, the column is maintained at a temperature of 100
) equipped with a thermal conductivity detector. Under typical conditions, the instrument contains a 4-mm × 3-m stainless steel column packed with liquid phase G11 on support S1A, the column is maintained at a temperature of 100 to 110
to 110 , the injection port is maintained at about 150
, the injection port is maintained at about 150 , and dry helium is used as the carrier gas at a flow rate of about 60 mL per minute. Calculate the percentage purity by dividing 100 times the area of the methoxyflurane peak by the sum of all the areas in the chromatogram.
, and dry helium is used as the carrier gas at a flow rate of about 60 mL per minute. Calculate the percentage purity by dividing 100 times the area of the methoxyflurane peak by the sum of all the areas in the chromatogram.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
| Monograph | Daniel K. Bempong, Ph.D.
Senior Scientist 1-301-816-8143 |
(MDPS05) Monograph Development-Pulmonary and Steroids |
| Reference Standards | Lili Wang, Technical Services Scientist 1-301-816-8129 RSTech@usp.org |
USP32–NF27 Page 2933
Pharmacopeial Forum: Volume No. 31(5) Page 1388