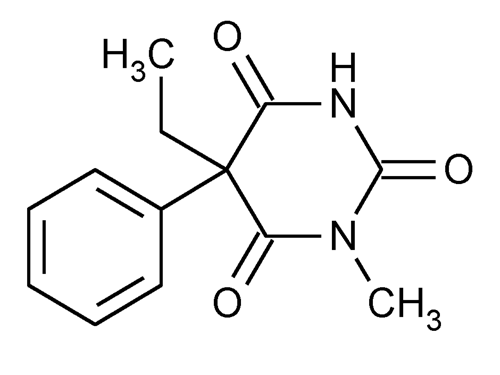
Mephobarbital
2,4,6(1H,3H,5H)-Pyrimidinetrione, 5-ethyl-1-methyl-5-phenyl-.
5-Ethyl-1-methyl-5-phenylbarbituric acid
» Mephobarbital contains not less than 98.0 percent and not more than 100.5 percent of C13H14N2O3, calculated on the dried basis.
Packaging and storage—
Preserve in well-closed containers.
Identification—
B:
Boil about 200 mg with 10 mL of 1 N sodium hydroxide: ammonia is evolved.
C:
Shake about 60 mg with 5 mL of sodium hydroxide solution (1 in 500), and filter. To a 1-mL portion of the filtrate add 3 drops of mercuric nitrate TS: a white precipitate is formed, and it is soluble in 6 N ammonium hydroxide. To another 1-mL portion of the filtrate add silver nitrate TS, dropwise: a white precipitate is formed, and it dissolves readily in 6 N ammonium hydroxide.
Loss on drying  731
731 —
Dry it at 105
—
Dry it at 105 for 4 hours: it loses not more than 1.0% of its weight.
for 4 hours: it loses not more than 1.0% of its weight.
Residue on ignition  281
281 :
not more than 0.1%.
:
not more than 0.1%.
Assay—
Dissolve about 500 mg of Mephobarbital, accurately weighed, in 50 mL of dimethylformamide in a 200-mL flask. Add 4 drops of thymolphthalein TS, and titrate with 0.1 N lithium methoxide in toluene VS, using a magnetic stirrer and a cover for the flask to protect against atmospheric carbon dioxide. Perform a blank determination, and make any necessary correction. Each mL of 0.1 N lithium methoxide is equivalent to 24.63 mg of C13H14N2O3.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
Chromatographic Column—
| Topic/Question | Contact | Expert Committee |
| Monograph | Ravi Ravichandran, Ph.D.
Senior Scientist 1-301-816-8330 |
(MDPP05) Monograph Development-Psychiatrics and Psychoactives |
| Reference Standards | Lili Wang, Technical Services Scientist 1-301-816-8129 RSTech@usp.org |
USP32–NF27 Page 2884
Pharmacopeial Forum: Volume No. 30(5) Page 1634
Chromatographic columns text is not derived from, and not part of, USP 32 or NF 27.