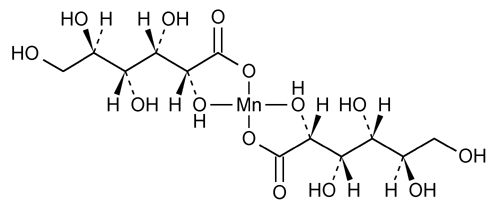
Manganese Gluconate
Bis(d-gluconato-O1,O2) manganese.
Manganese d-gluconate (1:2).
Dihydrate 481.27
» Manganese Gluconate is dried or contains two molecules of water of hydration. It contains not less than 98.0 percent and not more than 102.0 percent of C12H22MnO14, calculated on the anhydrous basis.
Packaging and storage—
Preserve in well-closed containers.
Labeling—
The label indicates whether it is the dried or the dihydrate form.
Identification—
A:
A solution (1 in 20) responds to the tests for Manganese  191
191 .
.
Water, Method I  921
921 (where labeled as the dried form):
between 3.0% and 9.0%, the determination being performed by stirring the mixture containing the Test preparation, maintained at a temperature of 50
(where labeled as the dried form):
between 3.0% and 9.0%, the determination being performed by stirring the mixture containing the Test preparation, maintained at a temperature of 50 , for 30 minutes before titrating with the Reagent: where labeled as the dihydrate it is between 6.0% and 9.0%.
, for 30 minutes before titrating with the Reagent: where labeled as the dihydrate it is between 6.0% and 9.0%.
Chloride  221
221 —
A 1.0-g portion shows no more chloride than corresponds to 0.70 mL of 0.020 N hydrochloric acid (0.05%).
—
A 1.0-g portion shows no more chloride than corresponds to 0.70 mL of 0.020 N hydrochloric acid (0.05%).
Sulfate  221
221 —
A 2.0-g portion shows no more sulfate than corresponds to 4.0 mL of 0.020 N sulfuric acid (0.2%).
—
A 2.0-g portion shows no more sulfate than corresponds to 4.0 mL of 0.020 N sulfuric acid (0.2%).
Limit of lead—
[note—For the preparation of all aqueous solutions and for the rinsing of glassware before use, employ water that has been passed through a strong-acid, strong-base, mixed-bed ion-exchange resin before use. Select all reagents to have as low a content of lead as practicable, and store all reagent solutions in containers of borosilicate glass. Cleanse glassware before use by soaking in warm 8 N nitric acid for 30 minutes and by rinsing with deionized water.]
Ascorbic acid–sodium iodide solution—
Dissolve 20 g of ascorbic acid and 38.5 g of sodium iodide in water in a 200-mL volumetric flask, dilute with water to volume, and mix.
Trioctylphosphine oxide solution—
[Caution—This solution causes irritation. Avoid contact with eyes, skin, and clothing. Take special precautions in disposing of unused portions of solutions to which this reagent is added.
] Dissolve 5.0 g of trioctylphosphine oxide in 4-methyl-2-pentanone in a 100-mL volumetric flask, dilute with the same solvent to volume, and mix.
Standard solution and Blank—
Transfer 5.0 mL of Lead Nitrate Stock Solution, prepared as directed in the test for Heavy Metals  231
231 , to a 100-mL volumetric flask, dilute with water to volume, and mix. Transfer 2.0 mL of the resulting solution to a 50-mL volumetric flask. To this volumetric flask and to a second, empty 50-mL volumetric flask (Blank) add 10 mL of 9 N hydrochloric acid and about 10 mL of water. To each flask add 20 mL of Ascorbic acid–sodium iodide solution and 5.0 mL of Trioctylphosphine oxide solution, shake for 30 seconds, and allow to separate. Add water to bring the organic solvent layer into the neck of each flask, shake again, and allow to separate. The organic solvent layers are the Blank and the Standard solution, and they contain 0.0 µg and 2.0 µg of lead per mL, respectively.
, to a 100-mL volumetric flask, dilute with water to volume, and mix. Transfer 2.0 mL of the resulting solution to a 50-mL volumetric flask. To this volumetric flask and to a second, empty 50-mL volumetric flask (Blank) add 10 mL of 9 N hydrochloric acid and about 10 mL of water. To each flask add 20 mL of Ascorbic acid–sodium iodide solution and 5.0 mL of Trioctylphosphine oxide solution, shake for 30 seconds, and allow to separate. Add water to bring the organic solvent layer into the neck of each flask, shake again, and allow to separate. The organic solvent layers are the Blank and the Standard solution, and they contain 0.0 µg and 2.0 µg of lead per mL, respectively.
Test solution—
Add 1.0 g of Manganese Gluconate, 10 mL of 9 N hydrochloric acid, about 10 mL of water, 20 mL of Ascorbic acid–sodium iodide solution, and 5.0 mL of Trioctylphosphine oxide solution to a 50-mL volumetric flask, shake for 30 seconds, and allow to separate. Add water to bring the organic solvent layer into the neck of the flask, shake again, and allow to separate. The organic solvent layer is the Test solution.
Procedure—
Concomitantly determine the absorbances of the Blank, Standard solution, and Test solution at the lead emission line at 283.3 nm, with a suitable atomic absorption spectrophotometer (see Spectrophotometry and Light-Scattering  851
851 ) equipped with a lead hollow-cathode lamp and an air–acetylene flame, using the Blank to set the instrument to zero. In a suitable analysis, the absorbance of the Standard solution and the absorbance of the Blank are significantly different: the absorbance of the Test solution does not exceed that of the Standard solution (0.001%).
) equipped with a lead hollow-cathode lamp and an air–acetylene flame, using the Blank to set the instrument to zero. In a suitable analysis, the absorbance of the Standard solution and the absorbance of the Blank are significantly different: the absorbance of the Test solution does not exceed that of the Standard solution (0.001%).
Heavy metals  231
231 —
Dissolve 1 g in 10 mL of water, add 6 mL of 3 N hydrochloric acid, and dilute with water to 25 mL: the limit is 20 µg per g.
—
Dissolve 1 g in 10 mL of water, add 6 mL of 3 N hydrochloric acid, and dilute with water to 25 mL: the limit is 20 µg per g.
Reducing substances—
Transfer 1.0 g to a 250-mL conical flask, dissolve in 10 mL of water, and add 25 mL of alkaline cupric citrate TS. Cover the flask, boil gently for 5 minutes, accurately timed, and cool rapidly to room temperature. Add 25 mL of 0.6 N acetic acid, 10.0 mL of 0.1 N iodine VS, and 10 mL of 3 N hydrochloric acid, and titrate with 0.1 N sodium thiosulfate VS, adding 3 mL of starch TS as the endpoint is approached. Perform a blank determination, omitting the specimen, and note the difference in volumes required. Each mL of the difference in volume of 0.1 N sodium thiosulfate consumed is equivalent to 2.7 mg of reducing substances (as dextrose): the limit is 1.0%.
Assay—
Dissolve about 700 mg of Manganese Gluconate, accurately weighed, in 50 mL of water. Add 1 g of ascorbic acid, 10 mL of ammonia-ammonium chloride buffer TS, and 0.1 mL of eriochrome black TS, and titrate with 0.05 M edetate disodium VS until the solution is deep blue in color. Each mL of 0.05 M edetate disodium is equivalent to 22.26 mg of C12H22MnO14.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
Chromatographic Column—
| Topic/Question | Contact | Expert Committee |
| Monograph | Curtis Phinney
1-301-816-8540 |
(DSN05) Dietary Supplements - Non-Botanicals |
| Reference Standards | Lili Wang, Technical Services Scientist 1-301-816-8129 RSTech@usp.org |
USP32–NF27 Page 2846
Pharmacopeial Forum: Volume No. 29(3) Page 636
Chromatographic columns text is not derived from, and not part of, USP 32 or NF 27.