Iron Sucrose Injection
» Iron Sucrose Injection is a sterile, colloidal solution of ferric hydroxide in complex with Sucrose in Water for Injection. It contains not less than 95.0 percent and not more than 105.0 percent of the labeled amount of iron. Sodium Hydroxide may be added to adjust the pH. It contains no antimicrobial agent, chelating agent, dextran, gluconate, or other added substances.
Packaging and storage—
Preserve in single-dose containers of Type I glass. Store at controlled room temperature. Do not freeze.
Labeling—
Label it to state that it is for intravenous use only. Label it to indicate that when administered by intravenous infusion, the Injection must be diluted with 0.9% Sodium Chloride Injection to a concentration of 0.5 to 2.0 mg of elemental iron per mL. Label it also to state the total osmolarity of the solution expressed in mOsmol per L.
Identification—
A:
Iron—To 2.5 mL of Injection add 17.5 mL of water and 5 mL of hydrochloric acid, mix, and heat for 5 minutes in a boiling water bath. Cool, add dropwise 13.5 N ammonium hydroxide until no further precipitation of ferric hydroxide occurs, and filter. Wash the precipitate with water to remove excess ammonium hydroxide, dissolve the precipitate in a minimum volume of 2 N hydrochloric acid, and add sufficient water to make a volume of 20 mL. To 3 mL of the solution so obtained add 1 mL of 2 N hydrochloric acid and 1 mL of potassium thiocyanate TS: the resulting solution (Solution 1) is red. To 1 mL of Solution 1 add 5 mL of amyl alcohol or ethyl ether, shake, and allow to stand: the organic layer is pink. To a separate 1-mL aliquot of Solution 1 add 2 mL of mercuric chloride TS: the red color is discharged [iron (III) salts].
B:
Sucrose—The retention time of the major peak in the chromatogram of the Assay preparation corresponds to that in the chromatogram of the Standard preparation, as obtained in the Assay for sucrose.
C:
Molecular weight determination—
Mobile phase—
Dissolve 7.12 g of dibasic sodium phosphate dihydrate, 5.52 g of monobasic sodium phosphate, and 0.40 g of sodium azide in 2 L of water.
System suitability solution—
Dissolve 200 mg of high molecular weight dextran and 100 mg of glucose in 20 mL of Mobile phase.
Standard solutions—
Transfer about 20 mg of each polysaccharide molecular weight standard (5,000–400,000 Da), accurately weighed, to separate 5-mL volumetric flasks. Add 4 mL of Mobile phase to each flask, and allow each aliquot to stand at or below 25 for a minimum of 12 hours. After the agglomerate particles of each Standard solution have swelled to their fullest extent, gently swirl each Standard solution until dissolved. [note—The chromatograms of freshly prepared Standard solutions regularly show a small, unidentified secondary peak following the main peak. Discard the Standard solutions if the secondary peak reaches half the height of the main peak.]
for a minimum of 12 hours. After the agglomerate particles of each Standard solution have swelled to their fullest extent, gently swirl each Standard solution until dissolved. [note—The chromatograms of freshly prepared Standard solutions regularly show a small, unidentified secondary peak following the main peak. Discard the Standard solutions if the secondary peak reaches half the height of the main peak.]
Test solution—
Transfer 5.0 mL of Injection to a 10-mL volumetric flask, dilute with Mobile phase to volume, and mix.
Chromatographic system (see Chromatography  621
621 )—
The liquid chromatograph is equipped with a refractive index detector maintained at a constant temperature of 45
)—
The liquid chromatograph is equipped with a refractive index detector maintained at a constant temperature of 45 and two 7.8-mm × 30-cm columns set up in series that contain packing L39 with pore sizes of 1000
and two 7.8-mm × 30-cm columns set up in series that contain packing L39 with pore sizes of 1000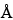 and 120
and 120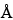 , respectively. The column temperatures are maintained at 45 ± 2
, respectively. The column temperatures are maintained at 45 ± 2 and the flow rate is about 0.5 mL per minute. Chromatograph the System suitability solution, and measure the peak areas as directed for Procedure: the resolution, R, between dextran and glucose is not less than 4.0. Chromatograph the Standard solutions, and measure the peak areas as directed for Procedure. Using a suitable program, plot the retention times of the Standard solutions and their molecular weights to generate a third order (cubic) calibration curve. The correlation coefficient obtained is not less than 0.98.
and the flow rate is about 0.5 mL per minute. Chromatograph the System suitability solution, and measure the peak areas as directed for Procedure: the resolution, R, between dextran and glucose is not less than 4.0. Chromatograph the Standard solutions, and measure the peak areas as directed for Procedure. Using a suitable program, plot the retention times of the Standard solutions and their molecular weights to generate a third order (cubic) calibration curve. The correlation coefficient obtained is not less than 0.98.
Procedure—
Separately inject equal volumes (about 25 µL) of each Standard solution, the System suitability solution, and the Test solution into the chromatograph, record the chromatograms, and measure the retention times and peak areas. The molecular weight of the complex is calculated from the calibration curve. The molecular weight distribution curve of the sample is sliced into fractions. Calculate the weight-average molecular weight, MW , as follows:
MW = 34,000–60,000 Da,
MN = not less than 24,000 Da, and
MW / MN = not more than 1.7.
S(AT MT)/SAT
and the number-average molecular weight, MN , as follows:
S(AT)/S(AT / MT)
in which AT is the area of each fraction of the sample distribution; and MT is the corresponding mean molecular weight of each fraction as determined from its retention time on the calibration curve. The molecular weight distribution curve obtained for the Injection conforms to the following parameters:
MW = 34,000–60,000 Da,
MN = not less than 24,000 Da, and
MW / MN = not more than 1.7.
Specific gravity  841
841 :
not less than 1.135 and not more than 1.165 at 20
:
not less than 1.135 and not more than 1.165 at 20 .
.
Bacterial endotoxins  85
85 :
not more than 3.7 USP Endotoxin Units per mg of iron contained in the Injection.
:
not more than 3.7 USP Endotoxin Units per mg of iron contained in the Injection.
Alkalinity—
Transfer 5 mL of Injection to a suitable vessel, and titrate with 0.1 N hydrochloric acid VS with constant stirring to a pH of 7.4. Record the volume of 0.1 N hydrochloric acid VS consumed, and calculate the alkalinity of the Injection as the volume of acid, in mL, consumed per mL of Injection. Not less than 0.5 mL and not more than 0.8 mL of 0.1 N hydrochloric acid VS is consumed per mL of Injection.
pH  791
791 :
between 10.5 and 11.1 at 20
:
between 10.5 and 11.1 at 20 .
.
Osmolarity  785
785 :
not less than 1150 mOsmol per L and not more than 1350 mOsmol per L for the Injection. The solution for test is prepared by diluting the Injection 1 in 10.
:
not less than 1150 mOsmol per L and not more than 1350 mOsmol per L for the Injection. The solution for test is prepared by diluting the Injection 1 in 10.
Absence of low-molecular weight Fe(II) and Fe(III) complexes—
In the polarograms obtained in the test for Limit of iron (II), no additional peaks are found.
Turbidity—
Transfer 0.5 g of Injection to a 150-mL beaker, add 100 mL of water, and with constant stirring adjust with 0.1 N hydrochloric acid VS to a pH of about 6.0. Remove the pH electrode from the solution. Adjust a light source such that the beam hits the beaker at a parallel angle about 2 cm below the surface of the liquid. The light must shine through to the surface, and the solution must not have any turbidity. Measurement must be carried out in as dark a room as possible. Slowly add 0.1 N hydrochloric acid VS, dropwise, until a slight but lasting turbidity develops. Record the pH of the solution as the turbidity point of the Injection: not less than 4.4 and not more than 5.3.
Particulate matter  788
788 —
Prepare a solution of Injection (1 in 40) using water that has been passed through a filter having a 1.2-µm or finer porosity: meets the requirements for Light Obscuration Particle Count Test for small-volume injections.
—
Prepare a solution of Injection (1 in 40) using water that has been passed through a filter having a 1.2-µm or finer porosity: meets the requirements for Light Obscuration Particle Count Test for small-volume injections.
Limit of iron (II)—
Supplementary electrolyte solution—
Dissolve 15.0 g of sodium acetate in 100 mL of water, and adjust with 0.1 N acetic acid to a pH of 7.0.
Procedure—
Transfer a suitable amount of Supplementary electrolyte solution to a polarographic cell equipped with a mercury drop electrode. With the electrode submerged in the liquid, bubble nitrogen through the liquid for 5 minutes. Avoiding any undue exposure to air, immediately transfer a volume of Injection, accurately measured, equivalent to a concentration of about 20 to 120 µg of elemental iron per mL, to the polarographic cell. [note—The sample must be analyzed immediately upon opening the container.] Record the polarogram from 0 mV and –1700 mV. The iron (III)/iron (II) peak is detected at –750 ± 50 mV and the iron (II)/iron (0) peak is detected at –1400 ± 50 mV. Measure the iron (II)/iron (III) peak responses obtained from the polarogram, and perform a blank determination. Calculate the iron (II) content, in % w/v, in the volume of Injection taken by the formula:
[1– (2/R)] × Fe
in which R is the peak response ratio of iron (II) to iron (III); and Fe is the total iron concentration, in % w/v, of the Injection. Not more than 0.4% (w/v) of iron (II) is found.
Content of chloride—
Transfer about 12 g of Injection, accurately weighed, into a 50-mL beaker. Add 40 mL of water, 0.3 mL of 65% nitric acid, and, while stirring, titrate with 0.01 N silver nitrate VS, determining the endpoint potentiometrically with silver–glass electrodes. Calculate the chloride content, in mg, of Injection taken. Each mL of 0.01 N silver nitrate consumed is equal to 0.3545 mg of chloride (Cl). The chloride content of the Injection is not less than 0.012% and not more than 0.025%.
Other requirements—
It meets the requirements under Injections  1
1 .
.
Assay for sucrose—
Mobile phase—
Prepare a mixture of acetonitrile and water (79:21).
Standard preparations—
Dissolve an accurately weighed quantity of USP Sucrose RS in water, and quantitatively dilute with water to obtain solutions having known concentrations of about 13, 16, 18, 21, and 23 mg of sucrose per mL.
Assay preparation—
Transfer about 1.875 g of Injection, accurately weighed, into a 25-mL flask, add 1.25 mL of water, and mix. Add 1.25 mL of a monobasic sodium phosphate solution, prepared by dissolving 30 g in 50 mL, and mix. Allow the resulting solution to stand for 10 minutes to precipitate the colloidal ferric hydroxide. Dilute with water to volume, and mix. Centrifuge this solution at 3000 rpm for 15 minutes. Pass the resulting solution through a filter, discarding the first 2 mL of the filtrate.
Chromatographic system (see Chromatography  621
621 )—
The liquid chromatograph is equipped with a column compartment and a refractive index detector each maintained at a controlled temperature between 20
)—
The liquid chromatograph is equipped with a column compartment and a refractive index detector each maintained at a controlled temperature between 20 and 25
and 25 (±2
(±2 ) and a 4-mm × 25-cm column that contains packing L8. The flow rate is about 2.0 mL per minute. Chromatograph the Standard preparations, and measure the peak areas as directed for Procedure. The correlation coefficient obtained from the linear regression of the Standard preparations is not less than 0.998. [note—The retention time for sucrose is about 8 minutes.]
) and a 4-mm × 25-cm column that contains packing L8. The flow rate is about 2.0 mL per minute. Chromatograph the Standard preparations, and measure the peak areas as directed for Procedure. The correlation coefficient obtained from the linear regression of the Standard preparations is not less than 0.998. [note—The retention time for sucrose is about 8 minutes.]
Procedure—
Separately inject equal volumes (about 20 µL) of each Standard preparation and the Assay preparation into the chromatograph, record the chromatograms, and measure the areas for the major peaks. Plot the peak area for each Standard preparation versus concentration, in mg per mL, of sucrose, and draw the straight line best fitting the five plotted points. From the graph so obtained, determine the concentration, in mg per mL, of sucrose in the Assay preparation. Calculate the quantity, in mg, of sucrose in each mL of Injection taken by the formula:
CDG/W
in which C is the concentration, in mg per mL, of sucrose in the Assay preparation; D is the dilution volume of the Assay preparation; G is the density, in g per mL, of Injection taken; and W is the weight, in g, of Injection taken. It contains not less than 260 mg and not more than 340 mg of sucrose per mL.
Assay for iron—
Iron stock solution—
Transfer about 350 mg of ferrous ammonium sulfate, accurately weighed, to a 1000-mL volumetric flask, add water to dissolve, dilute with water to volume, and mix to obtain a solution having a concentration of about 50 µg of iron per mL.
Calcium chloride solution—
Transfer 2.64 g of calcium chloride to a 1000-mL volumetric flask, add 500 mL of water, and swirl to dissolve. Add 5.0 mL of hydrochloric acid, and dilute with water to volume.
Standard preparations—
To separate 50-mL volumetric flasks transfer 2.0, 4.0, 6.0, 8.0, and 10.0 mL of Iron stock solution. Dilute each flask with Calcium chloride solution to volume, and mix to obtain Standard preparations having known concentrations of about 2.0, 4.0, 6.0, 8.0, and 10.0 µg of iron per mL.
Assay preparation—
Using a “to contain” pipette, transfer 2.0 mL of Injection to a 100-mL volumetric flask. Rinse the pipette several times with Calcium chloride solution. Add 5 mL of hydrochloric acid, and swirl until the solution turns yellow. After the solution has cooled to room temperature, dilute with Calcium chloride solution to volume, and mix. Pipet 2.0 mL of this solution to a 100-mL volumetric flask, dilute with Calcium chloride solution to volume, and mix to obtain a solution with a theoretical concentration of about 8.0 µg of iron per mL.
Procedure—
Concomitantly determine the absorbances of the Standard preparations and the Assay preparation at the iron emission line at 248.3 nm with a suitable atomic absorption spectrophotometer (see Spectrophotometry and Light-Scattering  851
851 ) equipped with an iron hollow-cathode lamp and air–acetylene flame, using Calcium chloride solution as a blank. Plot the absorbances for each Standard preparation versus concentration, in µg per mL, of iron and draw the straight line best fitting the five plotted points. From the graph so obtained, determine the concentration, in µg per mL, of iron in the Assay preparation. Calculate the quantity, in mg, of iron in each mL of the Injection taken by the formula:
) equipped with an iron hollow-cathode lamp and air–acetylene flame, using Calcium chloride solution as a blank. Plot the absorbances for each Standard preparation versus concentration, in µg per mL, of iron and draw the straight line best fitting the five plotted points. From the graph so obtained, determine the concentration, in µg per mL, of iron in the Assay preparation. Calculate the quantity, in mg, of iron in each mL of the Injection taken by the formula:
5C/V
in which C is the concentration, in µg per mL, of iron in the Assay preparation; and V is the volume of Injection taken.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
Chromatographic Column—
| Topic/Question | Contact | Expert Committee |
| Monograph | Sujatha Ramakrishna, Ph.D.
Scientist 1-301-816-8349 |
(MDCV05) Monograph Development-Cardiovascular |
| Reference Standards | Lili Wang, Technical Services Scientist 1-301-816-8129 RSTech@usp.org |
|
| Radhakrishna S Tirumalai, Ph.D.
Senior Scientist 1-301-816-8339 |
(MSA05) Microbiology and Sterility Assurance |
USP32–NF27 Page 2690
Pharmacopeial Forum: Volume No. 28(4) Page 1130
Chromatographic columns text is not derived from, and not part of, USP 32 or NF 27.
